- Title
-
Collagen IX is Required for the Integrity of Collagen II Fibrils and the Regulation of Vascular Plexus Formation in Zebrafish Caudal Fins
- Authors
- Huang, C.C., Wang, T.C., Lin, B.H., Wang, Y.W., Johnson, S.L., and Yu, J.
- Source
- Full text @ Dev. Biol.
|
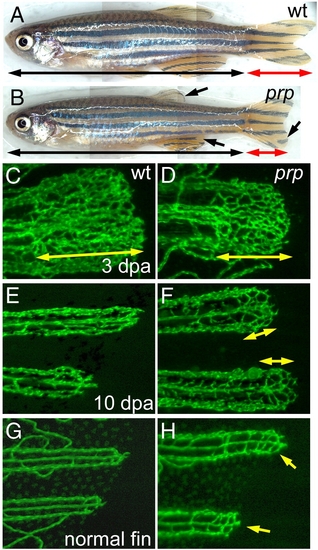
Persistent formation of vascular plexuses in ontogenetic and regenerating blood vessels in zebrafish prp mutants. (A) Wild-type (WT) and (B) prp adult zebrafish showing that the prp mutant has a normal body but smaller fins with rounded edges (arrows). (C) to (F) Regenerating experiments of fish after amputation. (C) A representative 3-day post-amputation (dpa) regenerating fin ray of the WT showing the vascular plexus at the fin tip (double-headed arrow). (D) Vascular plexuses seemed to form normally in prp 3-dpa regenerating fins (double-headed arrow). (E) At 10 dpa, fin vessels continued to regenerate without forming a plexus in the WT. (F) In the prp mutant, vascular plexuses (double-headed arrows), however, were still present in the 10-dpa regenerating fins. (G, H) WT and prp mutant fish. (G) The vascular plexus is normally not seen at the tips of normal WT fins. (H) In contrast, at almost every tip of a normal prp fin, a plexus-like vascular network could easily be detected (arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
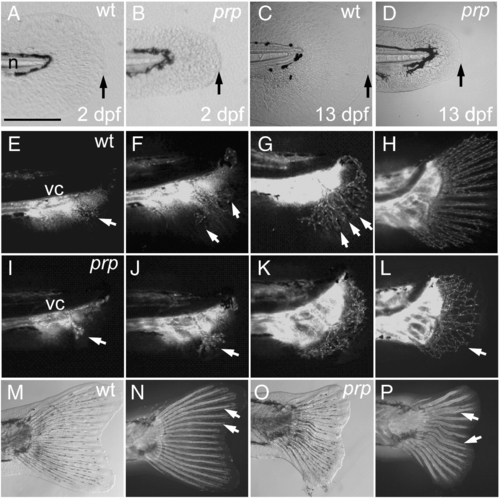
Small finfold and vascular plexus in young caudal fins of prp mutants. (A) Wild-type (WT) and (B) prp 2 day post-fertilization (dpf) embryos showing the small-finfold phenotype in prp. (C) WT and (D) prp larvae, at ∼ 13 dpf, showing the small-finfold phenotype throughout the larval stage in prp. Arrows in panels A–D denote the distal edges of the caudal finfolds. n, notochord. (E–H) Time lapse images of the developing vasculature between around 20 and 40 dpf in a WT caudal fin. (E) Fin vessels (arrow) growing from the posterior ends of axial vessels located below the vertebral column (vc). (F) Pioneer vessels (arrows) branching and growing dorsally and ventrally. (G) Fin vessels were organized into a radial pattern (arrows) soon after the vessels grow into the caudal fin. (H) Within a week, the mature 16–18-ray pattern of the fin vasculature was established. (I–L) Early development of the caudal fin vasculature in a representative prp mutant. (I, J) Pioneer fin vessels (arrows) growing out and branching normally in prp fish. (K, L) Instead of forming a radial pattern, the fin vessels of the prp mutant formed a large vascular plexus which expanded into the entire caudal fin (arrow in L). (M, bright light; N, fluorescence) In WT fish, the bony fin rays or lepidotrichia were stained by calcein fluorescent dye (arrows; Du et al., 2001) and were organized into a radial pattern associated with the vessels. (O, bright light; P, fluorescence) Fin rays of the prp mutant were often curved and juxtaposed and even fused (arrows). Anterior, left; dorsal top. Scale bars, 150 μm for panels A–D. EXPRESSION / LABELING:
PHENOTYPE:
|
|
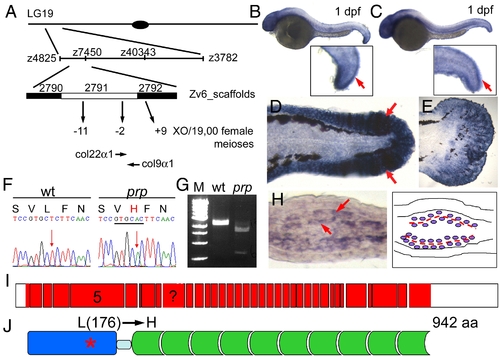
prp encodes the zebrafish col9α1. (A) Positional cloning of prp. Top, Chromosomal view of the location of prp. Areas are not drawn to scale. (B) col9α1 and (C) col22α1 were found to be expressed in 1-day post-fertilization (dpf) embryonic finfolds (arrows) by in situ hybridization. (D) col9α1 was also highly expressed in the finfold of a 2-dpf embryo and (E) the developing caudal fin of a 15-dpf larva. Anterior, left; dorsal top. (F) A T-to-A single nucleotide change was detected in the col9α1 gene of prp which created a restriction enzyme site for ApaLI (underlined) and caused a leucine (L) to change to histidine (H). (G) RT-PCR products covering the mutation site of prp were digested by ApaLI. M, DNA markers. (H) A cross-section of a regenerating fin showing that col9α1 was expressed in cells that sandwich the forming actinotrichia (arrows; further described in Fig. 5 and Fig. 6). (I) An intron–exon map showing that col9α1 is composed of at least 36 exons (red boxes) and untranslated regions (UTRs) (white boxes). Exon 5 encodes the thrombospondin domain, where the prp mutation is found, and is also what the morpholino was designed to delete. The exon with a question mark was not clearly resolved due to a lack of a genomic sequence in this region. (J) The protein structure of zebrafish COL9α1. The protein is 942-amino-acid long and contains a thrombospondin domain (blue) on the N terminus and a long stretch of glycine-X–Y repeats (green) separated by a small gap (light blue). The prp mutation resulted in an amino-acid change from leucine (176) to histidine in the thrombospondin domain of COL9α1. EXPRESSION / LABELING:
|
|
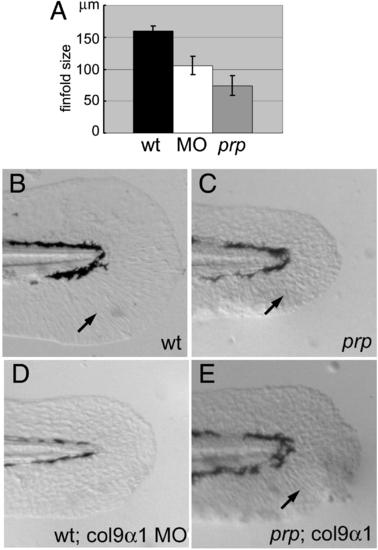
Col9α1 morpholino phenocopies the prp finfold phenotype while overexpression of col9α1 rescues prp. (A) A gene-specific morpholino (MO) targeting the splice site of col9α1 exon 5 caused the small-finfold phenotype in wild-type (WT) embryos. (B) The tail of a 2-day post-fertilization (dpf) uninjected WT embryo showing a normal finfold with radial actinotrichia (arrow). (C) A prp embryo showing a small finfold with short and sparse actinotrichia (arrow). (D) A WT embryo injected with the col9α1 MO developed the prp-like small-finfold and actinotrichia defects. (E) A prp embryo injected with the col9α1 plasmid developed a mosaic finfold with a prp-like portion in the dorsal half and a normal-looking portion in the ventral half where normal actinotrichia can be seen (arrow). Arrows, actinotrichia. Anterior, left; dorsal top. PHENOTYPE:
|
|
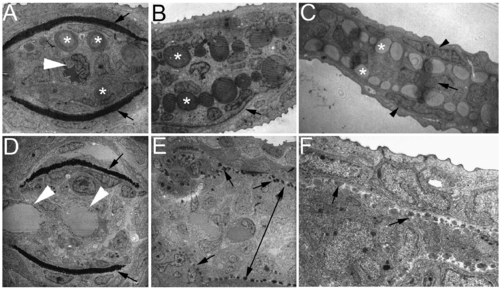
Ultrastructural analyses of zebrafish caudal fins by TEM. (A–C) Wild-type (WT) fin sections showing the structural organization of the fin tip. (A) In a proximal section, the lepidotrichia hemirays were stained dark (arrows) and actinotrichia are large bundles located inside near the hemirays (asterisks). A single artery can be seen in the center of the fin ray (arrowhead). (B) In a medial section, actinotrichial bundles were prominent (asterisks), but lepidotrichia were thin (arrow, only one side of the lepidotrichia is shown in this section). (C) In a distal section, the fin was much thinner and consisted of only the outer layers of the epidermis (arrowheads), numerous actinotrichia bundles (asterisks), and a one-cell layer of the mesenchyme (arrow). (D–F) Sections of a prp fin tip. (D) In a proximal section, the lepidotrichia hemirays are visible (arrows), but the actinotrichia were not present. Also in this section, two vessels can be observed in the intra-ray compartment (arrowheads) reflecting the plexus phenotype of prp. (E) In a medial section, actinotrichial bundles are visible but much smaller at almost the size of dots (arrows). Interestingly, the mesenchymal compartment was thicker in prp (double-headed arrow). (C) In a distal section, the actinotrichia were again small, and the intra-ray mesenchymal compartment was thick in prp. Note that one cannot directly compare the sizes of actinotrichia in (E) and (F) because they are of different magnifications. |
|
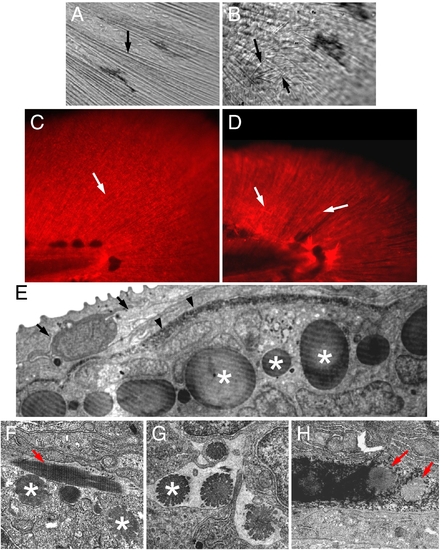
Actinotrichia defects in prp mutants. Bright light examination of (A) wild-type (WT) and (B) prp finfolds revealed short and mis-orientated actinotrichia in prp (arrows). (C, D) Immunostaining of zebrafish finfolds with a monoclonal antibody against human collagen II. (C) In the WT finfold, this antibody stained the actinotrichia (arrows). (D) In prp, however, the actinotrichia appeared less dense and were often crisscrossed (arrows). (E) Analyses with transmission electronic microscopy (TEM) showed that in the WT fin, actinotrichia (asterisks) were bundles of collagen matrices located beneath the epidermis (arrow) and lepidotrichia (arrowheads). (F–H) The actinotrichia of the prp mutant appeared broken and much smaller (asterisks), sometimes perpendicular (arrow in panel F), and misassembled within the lepidotrichia (arrow in panel H). PHENOTYPE:
|
|
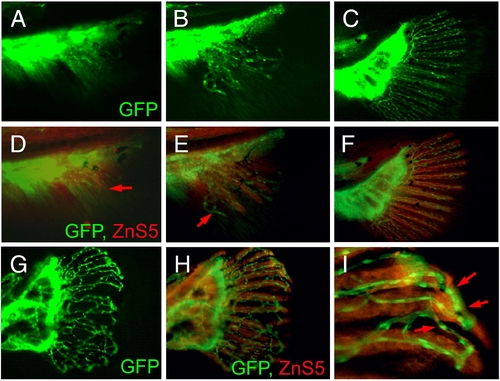
Vascular plexus causes wavy and fused fin rays in prp fish. (A–C) Blood vessel development in the caudal fin of wild-type (WT) fish. (D–F) Immunostaining of osteoblasts (red) with the ZnS5 monoclonal antibody of the same fins revealed that osteoblasts developed and were patterned after blood vessels as evidenced by areas where only blood vessels were present (arrows in panels D and E). (G) A prp fin showing the vascular plexus. (H) The same prp fin stained with ZnS5 (red) showing that the osteoblast pattern was closely associated with the blood vessel pattern. (I) High magnification of a representative area of (H) showing wavy and fused osteoblast rays along with the misconnected blood vessels (arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
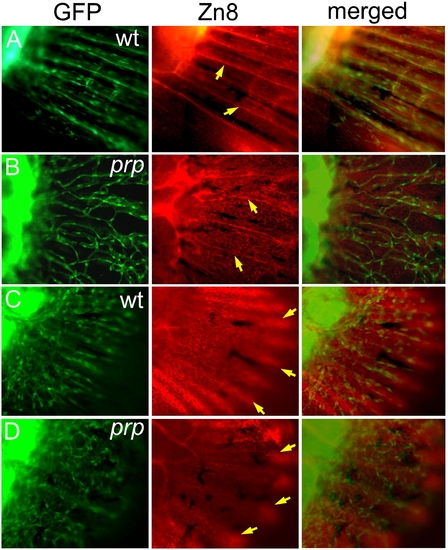
Independent developmental relationship between blood vessels, sensory axons, and the epidermis in a zebrafish caudal fin. (A) A wild-type (WT) fin stained with the Zn8 monoclonal antibody showing the sensory neuronal pattern in the fin. A single axon was found to extend along each fin ray (arrows). (B) In the prp mutant, the sensory axons maintained their ray pattern (arrows) and did not seem to be associated with the vascular plexus pattern. (C) A WT young fin stained with Zn8 and photographed with the focus on the epidermis showing that the epidermis formed a nice fin ray pattern (arrows). (D) A prp young fin showing that the epidermal pattern appeared relatively normal (arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
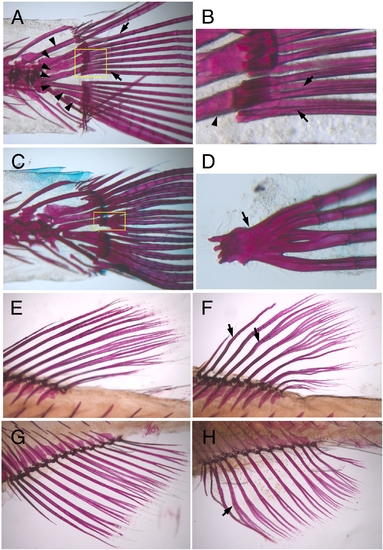
Wavy and fused lepidotrichia in prp mutants. Alizarin red staining of ossified bones (red) in zebrafish. Regions of the fin base are shown. (A, B) Wild-type (WT) adult zebrafish showing that lepidotrichia (arrows) were connected to the body through radial plates (arrowheads). (B) Close view of the lepidotrichia bases (boxed region of A) showing that the lepidotrichia were closely yet separately positioned (arrows). Arrowhead, radial plate. (C, D) The lepidotrichia in the prp mutant were often fused at the base (arrows). Although they managed to grow out as separate fin rays, they might be fused at more-distal points later in development (Fig. 2P). Alizarin red staining of WT dorsal (E) and anal (G) fins and prp dorsal (F) and anal (H) fins showing that the lepidotrichia of prp fins were wavy (arrows). |
|
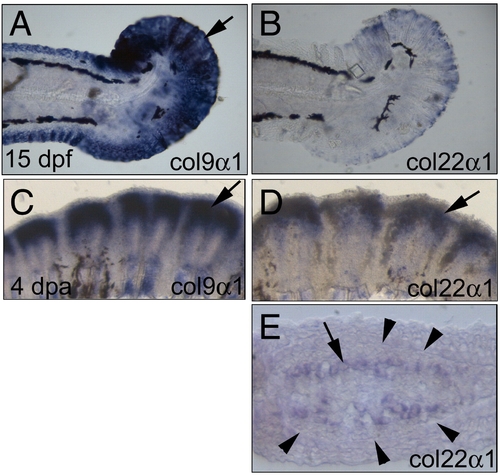
Expression patterns of col9α1 and col22α1 in the fins. (A) col9α1 was highly expressed in the 15-day post-fertilization (dpf) fin (arrow). (B) col22α1 was also weakly expressed in the 15-dpf fin. (C) In a 4-day post-amputation (dpa) regenerating fin, col9α1 was expressed in large amounts in the regenerating fin (arrow). (D) The 4-dpa regenerating fin also expressed high levels of col22α1 but in a relatively smaller domain (arrow). (E) Cross-section of (D) revealing that col22α1 was expressed in osteoblasts located inside the fin ray. The number of cells expressing col22α1 (arrows) was apparently smaller than that expressing col9α1 (compare with Fig. 3H). Arrowheads denote the thick basement membrane (seen as a line at this magnification) where the future fin ray will form in the regenerating fin. |
|
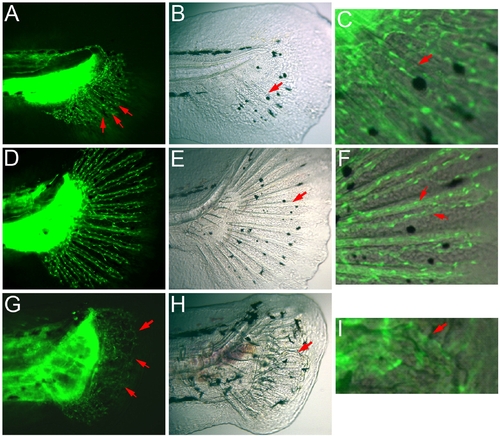
Association of fin rays and blood vessels in young caudal fins. (A–F) Wild-type (WT) young caudal fins. (A) At the early stage of caudal fin development when several radial rays of blood vessel had formed (arrows in A), only a few bony rays are visible (arrow in B). (C) Merged image of the fin ray area of (A) and (B) showing the association of fin rays and blood vessels (arrow). (D) At a later stage when the blood vessels had established the ray pattern, all of the bony rays are now obvious (arrow in E) and closely associated with blood vessels (arrows in the merged image in F). Note that there were only two vessels in each fin ray at this stage of development. (G–I) In the prp young caudal fin, the vascular plexus is prominent (arrows in G). Under bright light, the mispatterning of the fin rays is obvious (arrow in H). (I) Close-up of the merged image of the pointed fin ray in (H) showing the curvy fin ray associated with a curved blood vessel (arrow in I). |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 332(2), Huang, C.C., Wang, T.C., Lin, B.H., Wang, Y.W., Johnson, S.L., and Yu, J., Collagen IX is Required for the Integrity of Collagen II Fibrils and the Regulation of Vascular Plexus Formation in Zebrafish Caudal Fins, 360-370, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.