- Title
-
NeuroD regulates proliferation of photoreceptor progenitors in the retina of the zebrafish
- Authors
- Ochocinska, M.J., and Hitchcock, P.F.
- Source
- Full text @ Mech. Dev.
|
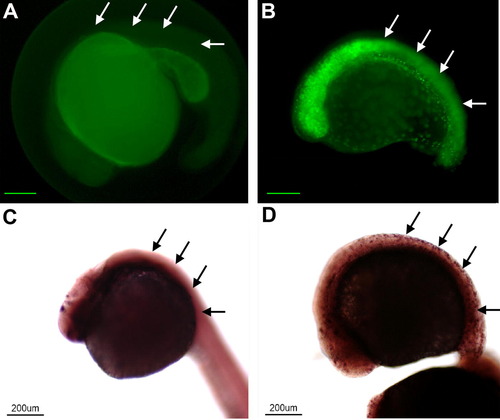
The NeuroD fusion protein is functional in vivo. Panels A (wt) and B (transgenic) illustrate embryos from the same clutch treated by heat shock at 15hpf and sacrificed at 25hpf. In the Tg(Hsp70/4:neuroDEGFP) EGFP is expressed throughout the body (cf. arrows in A and B, respectively). Panels C (wt) and D (transgenic) illustrate embryos from the same clutch treated by heat shock and processed for in situ hybridization for islet-1. In the transgenic embryo (D) islet-1 gene expression is induced throughout the body. Scale bars equal 200 μm in A and B. |
|
Induced neuroD promotes exit from the cell cycle exit in vivo, but does not cause cell death. Panel A illustrates a retina from a wt embryo treated with heat shock at 24hpf and labeled with BrdU and sacrificed at 48hpf. Note the extensive BrdU labeling throughout. Panel B is a retina from a transgenic embryo treated with heat shock at 24hpf and labeled with BrdU and sacrificed at 48hpf. Panel C is the retina illustrated in B, showing the BrdU labeling. Panel D is the digital overlay of panels B and C. Panel E illustrates histograms comparing the number of phosphohistone H3 immunopositive cells in the retinas of wt and transgenic embryos. Panel F illustrates histograms comparing the number of TUNEL-positive cells in the retinas of wt and transgenic embryos. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, circumferential marginal zone. Scale bar equals 50 μm. |
|
NeuroD increases the expression of cyclin inhibitors and inhibits the expression of cyclins. Panels A, C, E, G and I illustrate retinas from wt embryos heat-shocked at 24hpf, sacrificed at 48hpf and processed for in situ hybridization with probes for p27, p57, Cyclin D1, Cyclin B and Cyclin E, respectively. Panels B, D, F, H and J are transgenic embryos treated similarly. Scale bars equal 50 μm. |
|
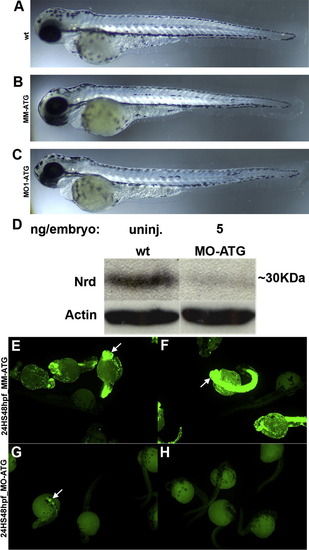
Morpholino oligonucleotides do not alter gross development, and specifically inhibit neuroD synthesis. Panels A–C illustrate wt, mismatch control and morphant embryos, respectively, at 72hpf. Note the similarity in body shape and eye size. Panel D is a Western blot of proteins from wt and morphant embryos. Panels E and F illustrate Tg(Hsp70/4:neuroDEGFP) embryos injected with either mismatch (E, F) or atg (G, H) morpholinos, treated with heat shock and 24hpf and photographed at 48hpf. Note the absence of EGFP fluorescence in the morphant embryos (G, H). |
|
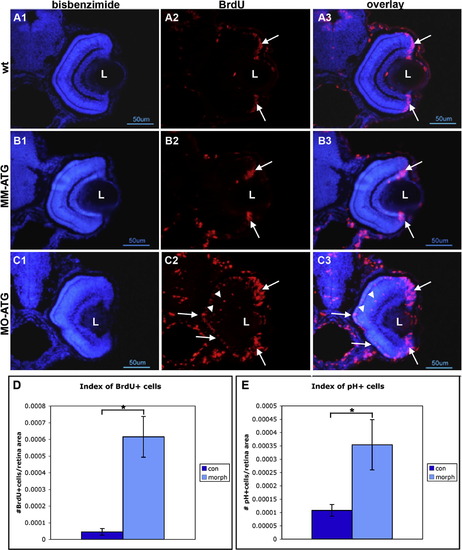
Cells fail to withdraw from the cell cycle following knock down of neuroD. Panels A, B and C illustrate retinas form wt, mismatch control and morphant fish, respectively, that were labeled with BrdU and sacrificed at 72hpf. Left-hand panels illustrate nuclear staining with bisbenzimide; middle panels illustrate BrdU labeling; right-hand panels are the respective digital overlays. L, lens. Panel D is histograms comparing the number of BrdU-labeled cells in control and morphant retinas. Panel E is histograms comparing the number of cells immunopositive for phosphohistone H3 in control and morphant retinas. Asterisks, p < 0.001. |
|
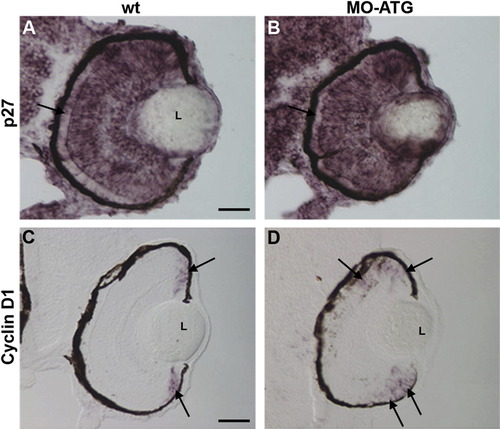
Cyclin D1 is upregulated in the absence of neuroD. Panels A and C illustrate wt retinas at 72hpf processed for in situ hybridization for p27and Cyclin D1, respectively. Panels B and D illustrate morphant retinas processed on the same microscope slides. L, lens. |
|
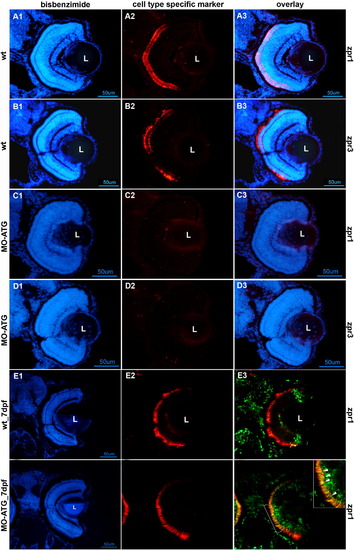
Photoreceptor differentiation is absent in morphant retinas at 72hpf and recovers in morphant retinas by 7 days post-fertilization. Rows A and B illustrate retinas from wt embryos at 72hpf stained for cone and rod photoreceptors, respectively. Rows C and D are retinas from morphants at 72hpf stained for cone and rod photoreceptors, respectively. Row E illustrates the retina from a wt animal labeled with BrdU at 72hpf, sacrificed at 7days post-fertilization (dpf) and labeled antibodies against BrdU (green) and the cone-specific marker, zpr1 (red). Row F illustrates the retina from a morphant labeled with BrdU at 72hpf, sacrificed at 7dpf and labeled with antibodies against BrdU (green) and the cone-specific marker, zpr1 (red). All left-hand panels illustrate nuclear staining with bisbenzimide. The middle panels are stained with markers for cones (zpr1) or rods (zpr3). The right-hand panels are digital overlays (E3 and F3). The scale bar in panel D1 corresponds to panels A–D and equals 50 μm. The scale bar in panel F1 corresponds to panels E–F and equals 50 μm. L, lens; wt, wild-type embryos; MO-ATG, embryos injected with translation-blocking morpholinos. |
|
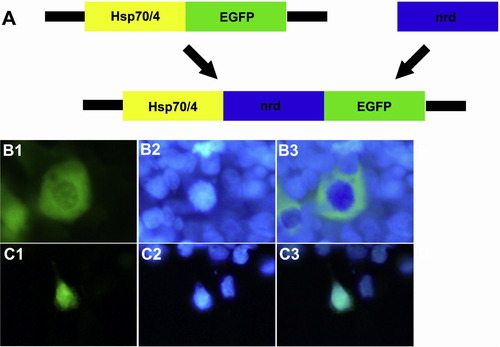
The construct encoding pHsp70/4:neuroDEGFP is transcribed in vitro. Panel A is a cartoon illustrating subcloning of the Hsp70/4:neuroDEGFP construct. The Hsp70/4:neuroDEGFP construct was assembled by subcloning the 1 kb coding region of the zebrafish neuroD gene into the Hsp70/4:EGFP vector. Panels B1-3 illustrate the localization of EGFP in the cytoplasm of HEK293 cells transfected with the empty Hsp70/4:EGFP construct. Panels C1-3 illustrate localization of EGFP in the nucleus of HEK293 cells transfected with the Hsp70/4:neuroDEGFP construct. Left-hand panels illustrate GFP fluorescence; Middle panels illustrate nuclear staining with bisbenzimide; right-hand panels are digital overlays. |
|
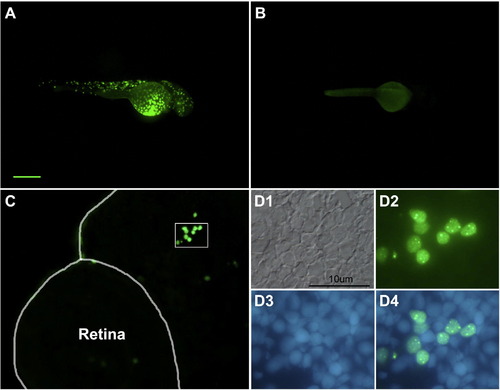
neuroDEGFP fusion protein is translated in vivo. Panel A illustrates a transient transgenic embryo treated with heat shock at 24hpf and photographed at 48hpf. Note the mosaic of GFP-labeled cells. Panel B is an uninjected control treated with heat shock. Panel C illustrates a section through the head and retina of a transient transgenic embryo at 48hpf. The enclosed area is illustrated at higher magnification (and rotated slightly) in D. Note the nuclear localization of the GFP. D1, Nomarski illumination; D2, GFP fluorescence; D3, nuclear staining with bisbenzimide; D4, digital overlay D2 and D3. The scale bar in A represents 200 μm for panels A and B and 50 μm for panel C. |
|
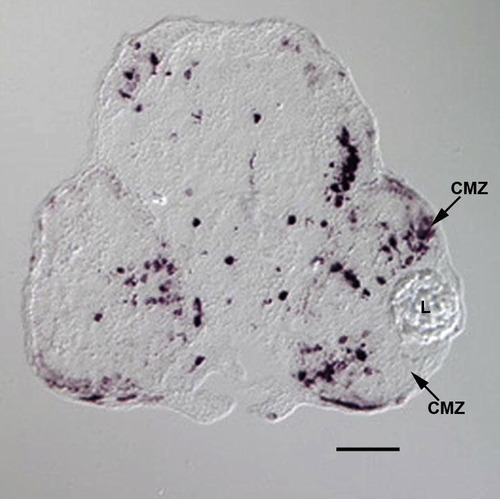
Ectopic expression of neuroD in a transient transgenic embryo. This panel illustrates in situ hybridization for neuroD in a transient transgenic embryo (see Fig. S2) treated with heat shock at 48hpf and sacrificed at 96hpf. Note the numerous labeled cells throughout the retina and head. Note the ectopic neuroD-expressing cells in the CMZ. CMZ, circumferential germinal zone. L, lens. Scale bar equals 50 μm. |
|
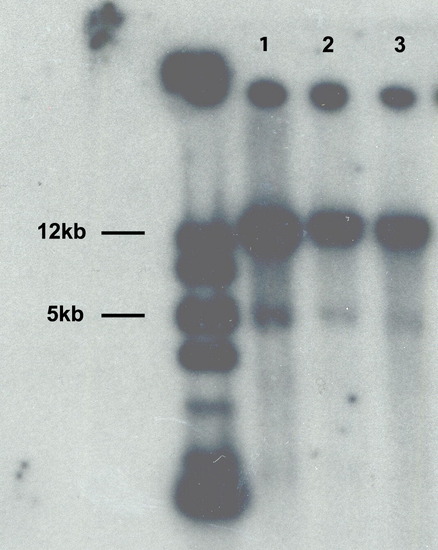
Southern blot analysis of lines transgenic for Hsp70/4:neuroDEGFP. Lanes 1–3 correspond to the three Tg(Hsp70/4:neuroDEGFP) lines. Lane 3 corresponds to the line selected for this study. Note the bands at 5 kb, which represent endogenous neuroD, and the intense bands at 12 kb, which represent the transgene. The left-most lane contains molecular weight markers. |
|
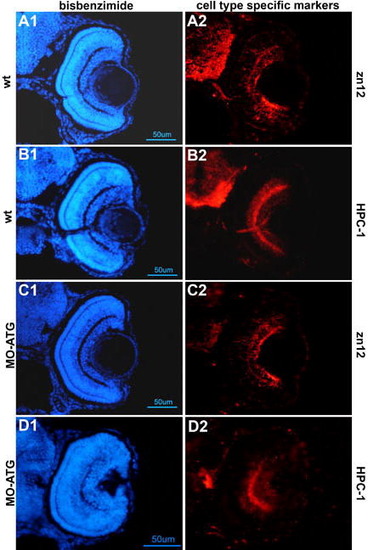
neuroD morphant retinas express markers of differentiated ganglion and amacrine cells. Panels A and B are wt embryos at 72hpf immunostained with zn12 and HPC-1 antibodies, respectively. Panels C and D are neuroD morphants treated similarly. Note that the inner retinal layers of the morphant retinas contain differentiated neurons. |
Reprinted from Mechanisms of Development, 126(3-4), Ochocinska, M.J., and Hitchcock, P.F., NeuroD regulates proliferation of photoreceptor progenitors in the retina of the zebrafish, 128-141, Copyright (2009) with permission from Elsevier. Full text @ Mech. Dev.