- Title
-
The pro-domain of the zebrafish Nodal-related protein Cyclops regulates its signaling activities
- Authors
- Tian, J., Andrée, B., Jones, C.M., and Sampath, K.
- Source
- Full text @ Development
|
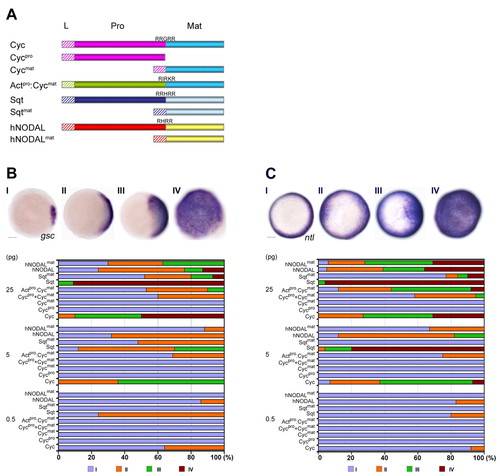
The Cyc pro-domain is essential for its activity. (A) Schematic representation of the Cyc precursor, Cycpro, Cycmat, Activinpro:Cycmat fusion, Sqt precursor, Sqtmat, hNODAL precursor and hNODALmat. L indicates the leader (hatched boxes), Pro and Mat indicate the pro-domain and the mature domain, respectively. Cleavage sites between the pro- and mature regions are shown for each precursor molecule. (B) Analysis of induction of gsc by overexpression of RNA encoding Cyc, Sqt and hNODAL. RNA was injected at 0.5, 5 and 25 pg doses into one-cell wild-type embryos and expression of gsc was examined at 50% epiboly. Animal pole views of embryos showing endogenous gsc expression (I), and mild (II) or massive (III and IV) expansion of the gsc expression domains. Embryos were assessed and counted accordingly. Percentages for each class are shown in the histogram. Scale bar: 100 μm. (C) Induction of ntl by overexpression of RNA encoding Cyc, Sqt and hNODAL. Animal pole views of embryos showing endogenous ntl expression (I), mild expansion (II) or massive expansion (III and IV) of the ntl expression domains. Embryos were assessed and scored accordingly. Percentages for each class are shown in the histogram. Unlike hNODALmat or Sqtmat, Cycmat has no discernible gsc- or ntl-inducing activity, and the Cyc pro-domain is required for its activity. Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
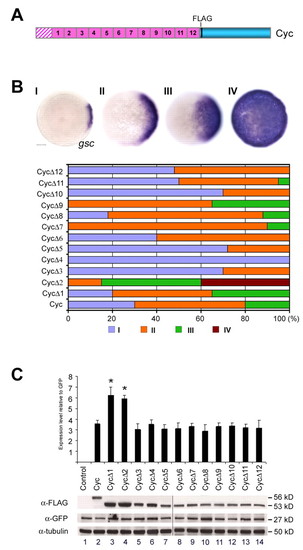
Specific regions within the Cyc pro-domain regulate its expression levels and activity. (A) Schematic representation of the Cyc precursor. The Cyc leader sequence is shown by the hatched box, the pro-domain in pink and the mature domain in blue. Regions 1-12 are 30 amino acid stretches in the pro-domain. (B) Analysis of induction of gsc in wild-type embryos injected with 1.25 pg aliquots of RNA encoding Cyc with deletions in regions 1-12 of the pro-domain. Some deletions, such as CycΔ2 and CycΔ9, have stronger gsc-inducing activity than wild-type Cyc, whereas some have either no detectable activity (CycΔ4) or reduced activity. Scale bar: 100 μm. (C) Expression of FLAG epitope-tagged versions of the various Cyc proteins. Immunoblots on extracts of HEK293T cells expressing FLAG-tagged Cyc proteins show more CycΔ1 and CycΔ2 protein than Cyc or the other deletions. The protein samples were loaded on two gels (lanes 1-7 and 8-14, respectively), and electrophoresed in parallel. Western blots of the input proteins are shown, detected with antibodies towards the FLAG epitope for the Cyc proteins, α-GFP for transfection control and α-Tubulin for loading controls. The graph shows normalized protein expression levels relative to the GFP control, from three independent experiments (mean±s.e.m.). Asterisks indicate significant differences; P<0.05 by paired Student's t test. A representative blot is shown. |
|
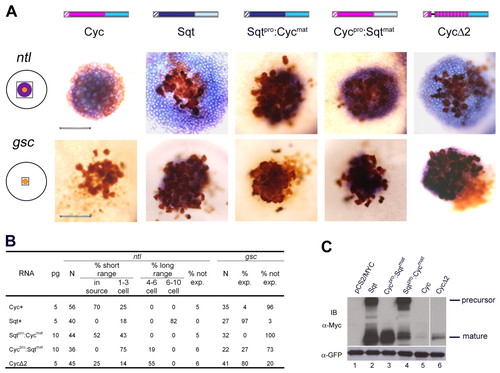
The Cyc pro-domain regulates processing, stability and signaling. (A) Schematic representation of Cyc, Sqt, Sqtpro:Cycmat, Cycpro:Sqtmat and CycΔ2. Hatched boxes indicate the leader sequences, the Cyc pro-domain is shown in pink, the Sqt pro-domain in dark blue, the Sqt mature domain in pale blue and the Cyc mature domain in mid-blue. RNA encoding the various Nodal-related proteins (5-10 pg) was injected into one-cell at the 64- to 128-cell stage together with a lineage tracer (brown). Range of signaling was examined by in situ hybridization to detect ntl (blue, top panel) and gsc (blue-brown, bottom panel) expression. All in situ panels show the boxed area indicated in the schematic of the embryo shown in the left. Expression of ntl is seen in all clones, whereas gsc expression is detected in the clones expressing Sqt, Cycpro:Sqtmat and CycΔ2, but not Cyc or Sqtpro:Cycmat. In comparison with Cyc, the range of activity of CycΔ2 is markedly expanded, as observed by expression of ntl, and CycΔ2 behaves similar to Sqt in inducing gsc. The Sqtpro:Cycmat fusion behaves like Cyc in the expression of both ntl and gsc, whereas ntl expression shows that Cycpro:Sqtmat has reduced signaling activity compared with Sqt. Expression of gsc is detected in some Cycpro:Sqtmat-injected embryos. All embryos were viewed from the animal pole. Scale bar: 100 μm. (B) Table summarizing ntl and gsc expression in the range of signaling assays. For gsc, the embryos were classified as with or without ectopic expression. The induction of ntl was classified as short range (expression in injected cells to three-cell diameters from injected source), long range (expression in cells that are 4-10 cell diameters from source) or not expressing. The amount of injected mRNA (pg), the total number of embryos examined (N) and the percentage of embryos in each category are indicated. (C) Supernatants from HEK293T cells expressing Myc-tagged Sqt, Cycpro:Sqtmat, Sqtpro:Cycmat, Cyc and CycΔ2 proteins. Both unprocessed (∼46 kDa) and processed (∼17 kDa) forms of Sqt are detected. The Cyc precursor is not detected, whereas substantial amounts of the Sqtpro:Cycmat precursor (∼45 kDa) are detected. For Cycpro:Sqtmat, no precursor is detected. Very low levels of processed Cycmat (∼16 kDa) is detected from supernatants of cell expressing Cyc, and marginally higher amounts of processed Cyc is detected in the CycΔ2 lane (∼16 kDa). The samples were electrophoresed on a SDS-PAGE gel; lane 6 (CycΔ2) is from a different part of the same gel. Expression of the transfection control GFP in cell lysates is shown in the lower panel. |
|
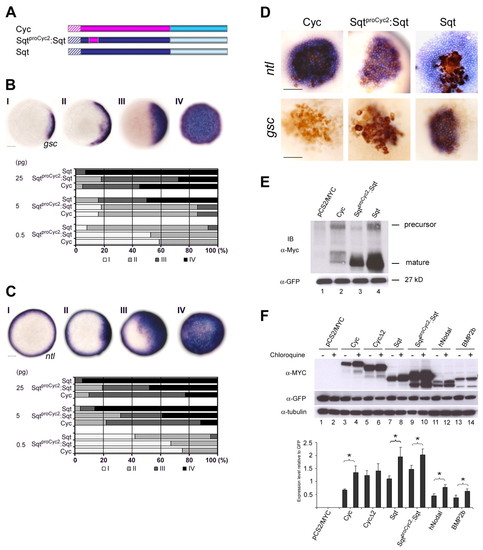
Region 2 of the Cyc pro-domain renders chimeric Sqt unstable, and targets the precursor for degradation. (A) Schematic representation of Cyc, Sqt and SqtproCyc2:Sqt. Hatched boxes indicate the leader sequences, the Cyc pro-domain is shown in pink, the Sqt pro-domain in dark blue, the Sqt mature domain in pale blue and the Cyc mature domain in mid-blue. (B,C) Analysis of induction of gsc (B) or ntl (C) in wild-type embryos injected at the one-cell stage with 0.5, 5 or 25 pg of RNA encoding Cyc, Sqt or SqtproCyc2:Sqt. Animal pole views of embryos at 50% epiboly showing endogenous gsc or ntl expression (I), and mild (II) or massive (III and IV) expansion of the gsc or ntl expression domains. Embryos were assessed and counted, and percentages for each class are shown in histograms. Scale bars: 100 μm. (D) 5 pg of RNA encoding Cyc, Sqt or SqtproCyc2:Sqt was injected into one cell at the 64- to 128-cell stage together with a lineage tracer (brown). Range of signaling was examined by in situ hybridization to detect ntl (blue, top panel) and gsc (blue-brown, bottom panel) expression. The SqtproCyc2:Sqt fusion behaves like Cyc, with its reduced ntl expression domain and the lack of gsc expression in most embryos (see Table S7 in the supplementary material). Scale bars: 100 μm. (E) Supernatants from HEK293T cells expressing Myc-tagged Sqt, Cyc and SqtproCyc2:Sqt proteins. Both unprocessed (∼46 kDa) and processed (∼17 kDa) forms of Sqt are detected. For SqtproCyc2:Sqt, the precursor is typically not detected and the processed Sqt (∼17 kDa) protein levels are reduced. The processed form of Cyc (∼16 kDa) is detected at very low levels. Supernatants from cells expressing pCS2Myc were used as negative control. GFP expression in the cell lysates was used to measure transfection efficiency. (F) Immunoblots to detect Myc-tagged Cyc (∼58 kDa), CycΔ2 (∼55 kDa), Sqt (∼46 kDa), SqtproCyc2:Sqt (∼46 kDa), hNODAL (∼41 kDa) or Bmp2b (∼48 kDa) in extracts of cells grown in the presence or absence of the lysosomal inhibitor chloroquine. CycΔ2 expression is not significantly altered by chloroquine treatment. By comparison, Cyc and the other proteins are stabilized by chloroquine. GFP was used as the transfection control and tubulin was used as the loading control. The histogram shows normalized protein expression levels relative to the GFP control, from three independent experiments (mean±s.e.m.). The asterisks (*) indicate significant differences (P<0.05 as determined by paired Student's t-test) between cells not treated versus those treated with the lysosomal inhibitor for 24 hours. A representative gel from three independent experiments is shown. |
|
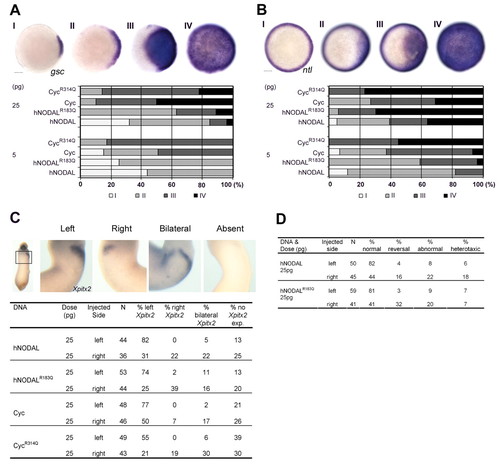
Mutation of a conserved arginine residue in hNODAL renders the protein more active and can induce left-right asymmetry defects. (A,B) CycR314Q and hNODALR183Q are more active than Cyc and hNODAL. Zebrafish embryos at the one-cell stage were injected with 5 or 25 pg of RNA encoding either Cyc, CycR314Q, hNODAL or hNODALR183Q. Expression of gsc (A) or ntl (B) was examined at 50% epiboly. The Arg-Gln mutations render CycR314Q and hNODALR183Q more active than the wild-type proteins. Animal pole views shown. Scale bar: 100 μm. (C) Overexpression of CycR314Q and hNODALR183Q in Xenopus embryos causes perturbations in left-sided expression of Xpitx2. Xenopus embryos at the four-cell stage were injected with 25 pg of DNA expression constructs encoding either Cyc, CycR314Q, hNODAL or hNODALR183Q in the dorsal-left or dorsal-right blastomeres and cultured until stage 28 and processed for in situ hybridization to detect Xpitx2 expression. Embryos were scored as left, right, bilateral or no Xpitx2 expression. Ventral views of stage 28 embryos with Xpitx2 expression in the cardiac primordia are shown. Injections of hNODALR183Q or CycR314Q can induce right-sided, bilateral or no Xpitx2 expression at a higher frequency than observed after injections of hNODAL or Cyc. (D) Gut looping is also perturbed by injection of hNODALR183Q. Xenopus embryos at the four-cell stage were injected with 25 pg of DNA encoding either hNODAL or hNODALR183Q in the dorsal-left or dorsal-right blastomeres and cultured until stage 45 and scored for the direction of intestinal coiling. Injected embryos manifested either normal, reversed or abnormal looping. In some embryos, heterotaxia of intestinal coiling and cardiac laterality was observed. Right-sided injections of hNODALR183Q increase the frequency of reversed intestinal coiling in comparison with hNODAL. |
|
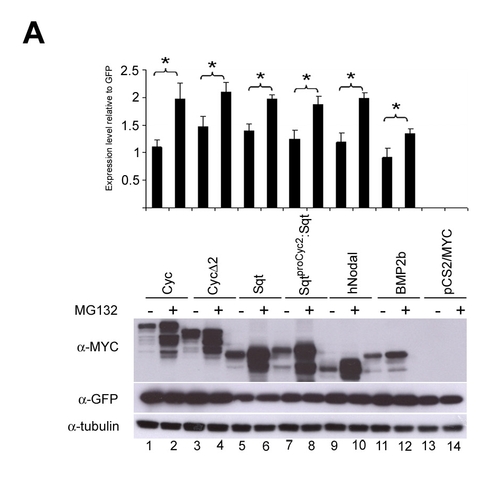
Nodal-related proteins are stabilized by 24-hour treatments with the proteasome inhibitor MG132. Immunoblots to detect Myc-tagged Cyc (∼58 kDa), Cyc 2 (∼55 kDa), Sqt (∼46 kDa), SqtproCyc2:Sqt (∼46 kDa), hNodal (∼41 kDa) or BMP2b (∼48 kDa) in extracts of cells grown in the presence or absence of the proteasome inhibitor MG132 for 24 hours show that all proteins are stabilized by treatment with MG132. Expression of GFP transfection control and Tubulin loading controls are shown. The graph shows normalized protein expression levels from three independent experiments, relative to the GFP control (mean±s.e.). The asterisk denotes significant difference (P<0.05 as determined by paired Student's t-test) between cells that were not treated versus those treated with the proteasome inhibitor for 24 hours. |