- Title
-
misty somites, a maternal effect gene identified by transposon-mediated insertional mutagenesis in zebrafish that is essential for the somite boundary maintenance
- Authors
- Kotani, T., and Kawakami, K.
- Source
- Full text @ Dev. Biol.
|
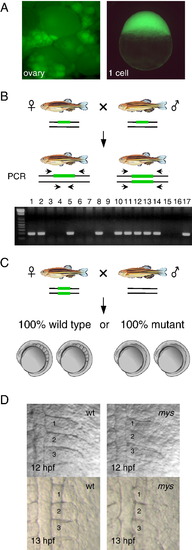
A scheme for isolation of maternal effect mutants by transposon-mediated gene trapping. (A) Identification of embryos that express GFP at the one cell stage. GFP expression in the ovary (left) and in fertilized eggs (right) (SAG20A). (B) Identification of fish homozygous for an insertion. Heterozygous male and female fish are crossed and the progeny are analyzed by PCR using primers that locate both sides of the insertion (green bars). The gel is an example of the analysis. A PCR product is amplified from heterozygous fish (lanes 1–2,5,8,10–14 and 17) but not from homozygous fish (lanes 3,4,6,7,9,15 and 16). (C) Homozygous female fish is crossed with wild type male fish and their progeny are analyzed for developmental defects. (D) The somite boundary phenotype at the 3- and 6-somite stages (12 hpf and 13 hpf). (left) Wild type embryos. (right) Embryos from SAG20A homozygous female fish. Numbers show the somite numbers. PHENOTYPE:
|
|
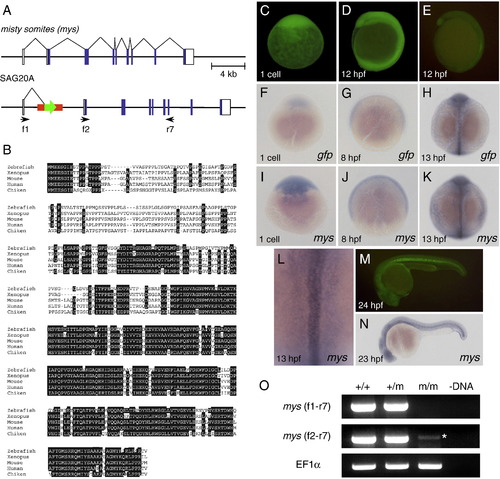
Characterization of the SAG20A insertion and the mys gene. (A) The structure of the wild type misty somites (mys) locus and the SAG20A insertion. White boxes indicate untranslated regions and blue boxes indicate coding regions of the mys gene. Arrows indicate primers used for RT-PCR. (B) Comparison of the amino acid sequences of the Mys proteins in zebrafish, Xenopus, mouse, human and chicken by using ClustalW. Identical amino acids are highlighted in white. (C, D) Maternal GFP expression at the one cell stage (C) and 12 hpf (D). Embryos were obtained from a cross between an SAG20A heterozygous female and a wild type male. The absence of the SAG20A insertion in this embryo was confirmed by PCR at later stages. (E) Zygotic GFP expression at 12 hpf. Embryos were obtained from a cross between an SAG20A heterozygous male and a wild type female. (F–L) In situ hybridization using the gfp probe (F–H) and the mys probe (I–L). (F) A one-cell stage embryo obtained from a cross between an SAG20A heterozygous female and a wild type male. 8 hpf (G) and 13 hpf (H) embryos obtained from an SAG20A heterozygous male and a wild type female. One-cell stage (I), 8 hpf (J), and 13 hpf (K, L) wild type embryos. mys is expressed in the notochord and weakly and diffusely in the paraxial mesoderm at 13 hpf. (M) Zygotic GFP expression at 24 hpf. Embryos were obtained from a cross between an SAG20A heterozygous male and a wild type female. (N) In situ hybridization of a wild type embryo at 23 hpf using the mys probe. (O) RT-PCR analysis of the mys transcript. RNA was prepared from wild type (+/+), heterozygous (+/m) and homozygous embryos (m/m) at 24 hpf and used for cDNA synthesis. (-DNA) A negative control without template DNA. RT-PCR was carried out by using f1 and r7 (top), f2 and r7 (middle), and the EF1&alpha primers (bottom: positive control). An asterisk indicates a weak transcript with an aberrant start site detected in homozygous embryos. |
|
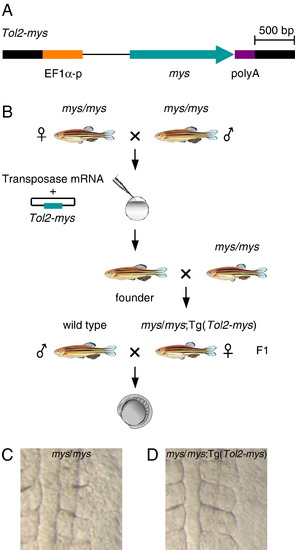
Rescue of the mys mutant phenotype by transgenesis. (A) The structure of the Tol2–mys construct. mys cDNA (mys) was cloned between the Xenopus EF1α promoter (EF1α-p) and the SV40 polyA signal (poly A). Thick black lines indicate the Tol2 sequence. (B) A transposon-donor plasmid containing Tol2–mys and the transposase mRNA were co-injected into fertilized eggs produced from mys homozygous parents. The injected fish were crossed with mys homozygous fish and transgenic fish were identified in the progeny. A female homozygous for the mys mutation (mys/mys) and carrying a single copy insertion of Tol2–mys was crossed with a wild type male, and the progeny were analyzed for the phenotype. (C, D) Dorsal views of embryos at the 6-somite stage. (C) Embryos from homozygous (mys/mys) female fish showed the obscure somite boundary phenotype. (D) Embryos from mys/mys; Tg(Tol2–mys) female fish showed distinct somite boundaries. PHENOTYPE:
|
|
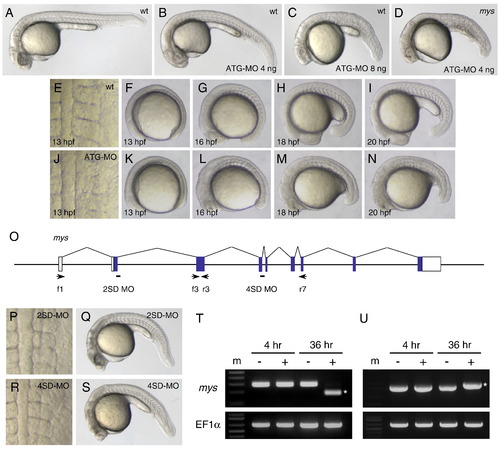
Inhibition of the mys activity by MO injection caused defects in the somite boundary formation. (A–C) Lateral views of embryos at 26 hpf. (A) A wild type embryo. (B) An ATG-MO injected embryo that showed the curved body phenotype (class I). (C) An ATG-MO injected embryo that showed the curved body and the somite boundary disappearance phenotypes (class II). (D) A mys mutant embryo at 26 hpf injected with 4 ng ATG-MO that showed the class II phenotype. (E–I) Dorsal and lateral views of a wild type embryo at 13 to 20 hpf. (J–N) Dorsal and lateral views of an ATG-MO injected embryo at 13 to 20 hpf. In the ATG-MO injected embryo, the obscure somite boundary phenotype is observed at 13 hpf (J), the distinct boundaries are formed at 16 hpf (L) and the boundaries disappear at 18 to 20 hpf (M, N). (O) Positions of 2SD-MO and 4SD-MO and primers. White boxes indicate untranslated regions and blue boxes indicate coding regions of the mys transcript. Arrows indicate positions and directions of primers used for RT-PCR. (P, Q) 2SD-MO injected embryos at 13 hpf and 26 hpf. (R, S) 4SD-MO injected embryos at 13 hpf and 26 hpf. The injected embryos showed the curved body phenotype but formed distinct somite boundaries. (T) RT-PCR using f1 and r3 (top) and using the EF1α primers (bottom). Embryos were injected with H2O (-) or 2SD-MO (+). RNA was prepared at 4 hpf and 36 hpf. (U) RT-PCR using f3 and r7 (top) and using the EF1α primers (bottom). Embryos were injected with H2O (-) or 4SD-MO (+). RNA was prepared at 4 hpf and 36 hpf. Asterisks indicate RT-PCR products of different sizes. SD-MO injection interfered with normal splicing of the zygotic mys transcript. (m) DNA size marker. PHENOTYPE:
|
|
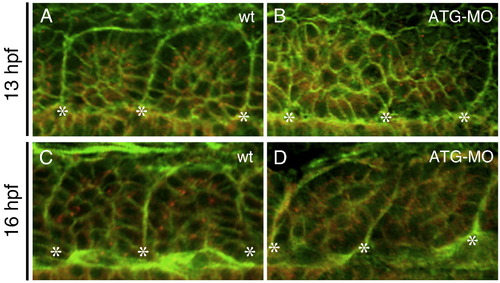
The mys-deficiency impaired epithelialization of somitic cells. (A–D) Confocal images of embryos stained with the anti-γ-Tubulin antibody (red) and Phalloidin (green). Dorsal views of a wild type embryo (A, C) and an ATG-MO injected embryo (B, D) at 13 hpf (the 6-somite stage) (A, B) or at 16 hpf (the 14-somite stage) (C, D). Asterisks indicate the positions of the somite boundaries. In wild type, somitic cells adjacent to the boundaries show a columnar shape and apical localization of the centrosomes. In the ATG-MO injected embryo, the somitic cells do not show the columnar shape and the centrosomes are distributed randomly in the cytoplasm. PHENOTYPE:
|
|
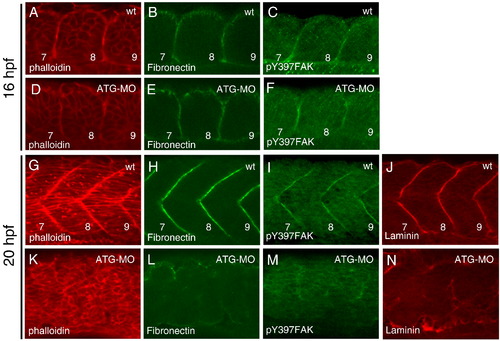
Disappearance of the somite boundaries in the mys-deficient embryo. Confocal images of wild type (A–C, G–J) and ATG-MO injected embryos (D–F, K–N). (A–F) Dorsal views of the 7th to 9th somite at 16 hpf (the 14-somite stage). (G–N) Lateral views of fast muscle cells of the 7th to 9th somite at 20 hpf (the 21-somite stage). Embryos were stained with phalloidin (A, D, G, K), anti-Fibronectin antibody (B, E, H, L), anti-phosphorylated FAK antibody (C, F, I, M) and anti-Laminin antibody (J, N). Numbers indicate the somite numbers. In the ATG-MO injected embryos, the somite boundaries disappeared at 20 hpf. PHENOTYPE:
|
|
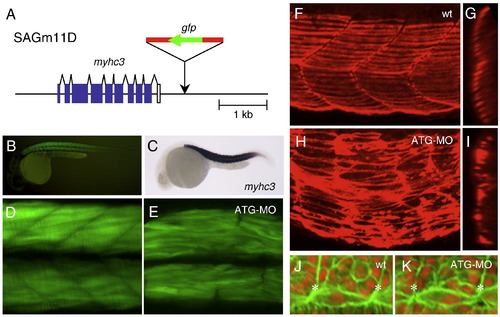
Abnormalities in the fast and slow muscle morphogenesis in the ATG-MO injected embryo. (A) The structure of the myhc3 locus and the SAGm11D insertion. T2KSAG was integrated downstream of the myhc3 gene. White boxes indicate untranslated regions and blue boxes indicate coding regions. (B) A lateral view of GFP expression in the SAGm11D embryo at 30 hpf. (C) A lateral view of a 24 hpf embryo hybridized with the myhc3 probe. (D) A confocal image of fast muscle fibers in an SAGm11D embryo at 36 hpf. (E) A confocal image of fast muscle fibers in an SAGm11D embryo injected with ATG-MO at 36 hpf. (F–I) Immunostaining using the S58 antibody that specifically detects slow muscle fibers. Confocal images of a wild type embryo (F, G) and an ATG-MO injected embryo at 32 hpf (H, I). (F, H) Lateral views of the surface area. (G, I) The same samples were rotated on 90° to generate transverse views. (J, K) Confocal images of somitic cells and adaxial cells located adjacent to the notochord. Dorsal views of a wild type embryo (J) and an ATG-MO injected embryo at 15 hpf (K). The notochord is at the bottom. Asterisks indicate the positions of the somite boundaries. The adaxial cells that elongate to span the length of the somite were formed both in wild type and the ATG-MO injected embryos. |
|
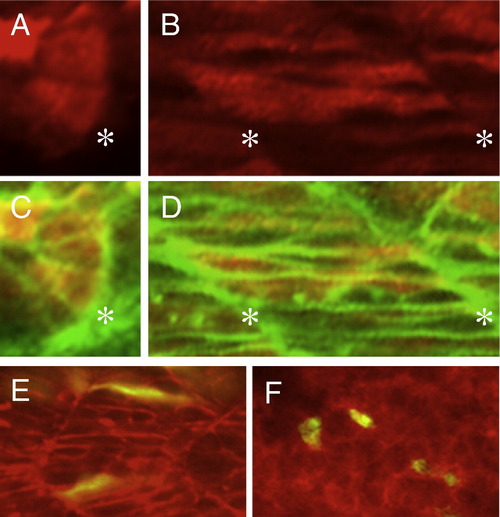
Immunostaining of the Flag–Mys fusion protein and cell transpolantation. (A–D) Confocal images of embryos injected with flag–mys mRNA and stained with the anti-Flag antibody (red) and Phalloidin (green). (A, C) Dorsal views of the somite at 16 hpf. (B, D) Lateral views of the somite at 20 hpf. (C, D) Merged images. Asterisks indicate the positions of the somite boundaries. The Flag–Mys protein was distributed throughout the cytoplasm. (E, F) Confocal images of lateral views of transplanted embryos at 20 hpf. (E) Cells from embryos injected with ATG-MO, rhodamine-dextran and GFP mRNA were transplanted into a wild type embryo. (F) Cells from embryos injected with rhodamine-dextran and GFP mRNA were transplanted into an ATG-MO injected embryo. The recipient embryos were stained with phalloidin (red) and transplanted cells were labeled in yellow. The ATG-MO injected cells were elongated in the wild type embryo (E) and the wild type cells showed rounded shapes in the ATG-MO injected embryo. |
|
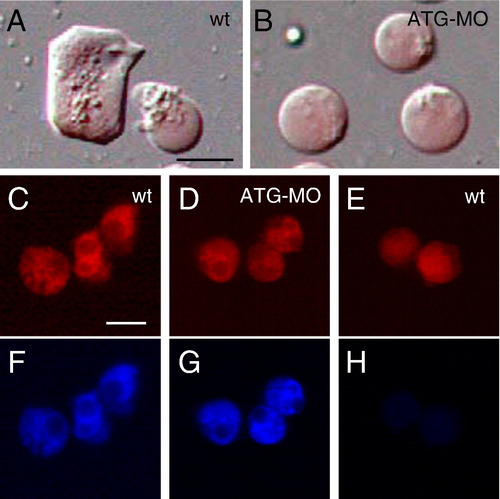
The mys-deficient cells decreased lamellipodia formation on Fibronectin. (A, B) The morphology of cells dissociated from 4 hpf embryos and incubated on Fibronectin for overnight. (A) Cells dissociated from wild type embryos. (B) Cells dissociated from the ATG-MO injected embryos. The embryos were injected with rhodamine-dextran (red). Lamellipodia formation is impaired in the ATG-MO injected cells. (C–H) Analysis of FAK activation. Wild type cells (C, F) and the ATG-MO injected cells (D, G) incubated on Fibronectin for overnight. (E, H) Wild type cells immediately after placed on Fibronectin. (C, D, E) Images of rhodamine-dextran. (F, G, H) Immunostaining using the anti-phosphorylated FAK antibody. FAK is activated in the ATG-MO injected cells. Scale bars show 20 μm. PHENOTYPE:
|
Reprinted from Developmental Biology, 316(2), Kotani, T., and Kawakami, K., misty somites, a maternal effect gene identified by transposon-mediated insertional mutagenesis in zebrafish that is essential for the somite boundary maintenance, 383-396, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.