- Title
-
Genetic analysis of two zebrafish patched homologues identifies novel roles for the hedgehog signaling pathway
- Authors
- Koudijs, M.J., den Broeder, M.J., Groot, E., and van Eeden, F.J.
- Source
- Full text @ BMC Dev. Biol.
|
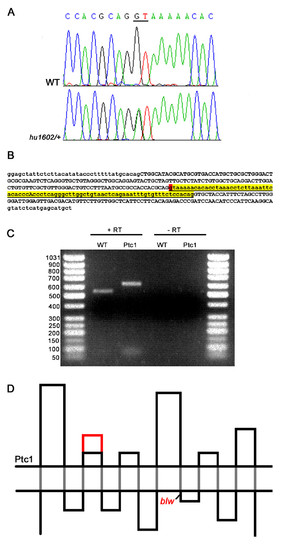
Identification and characterization of a splice-donor mutation in the zebrafish ptc1 gene. A splice donor mutation was identified in the first base pair of intron 10, changing the consensus sequence GT to AT, probably affecting splicing (A). The intron after exon 10, shown in lower case in yellow, contains 81 bp, and the splice donor position mutated in ptc1hu1602 is indicated in red (B). RT-PCR analysis confirms that splicing is affected as a result of the mutation, which extends the transcript with 81 bp compared to wild type (C). Schematic representation of the Ptc1 protein, showing the 12 transmembrane domains (black dots), and the extension of the second extracellular loop with 27 AA in red. The blw mutation is positioned directly after the eighth transmembrane domain of the protein (D). |
|
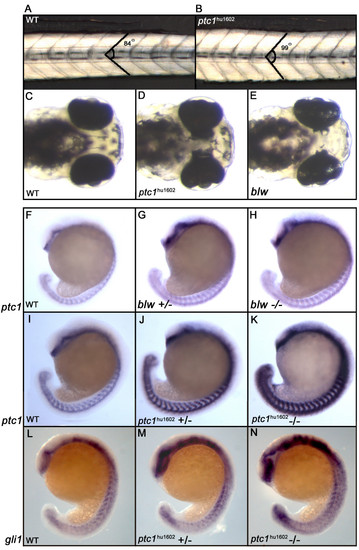
Phenotypic consequences of zebrafish ptc1 mutants. Homozygous ptc1hu1602 mutants show a subtle somite phenotype at 32 hpf, where the average angle of the somite becomes more obtuse (A,B). At 72 hpf, ptc1hu1602 mutants exhibit an eye phenotype where the pigmented epithelium is extended into the diencephalon. The similar phenotype described for the blw mutant is weaker compared to the ptc1hu1602 mutant (C-E). The expression level of ptc1, a general readout for Hh activity, shows a mild increase in the blw mutant compared to wild type (F-H). The ptc1hu1602 mutant shows a severely increased level of ptc1, where wild types, heterozygotes and mutants can be distinguished based on ptc1 levels (I-K). Additional to the difference in the strength of the eye phenotype, the activation of the pathway is significantly higher in ptc1hu1602 mutants compared to blw mutants. An increased expression level of gli1 confirms an activation of the Hh pathway in the ptc1hu1602 mutant (L-N). EXPRESSION / LABELING:
PHENOTYPE:
|
|
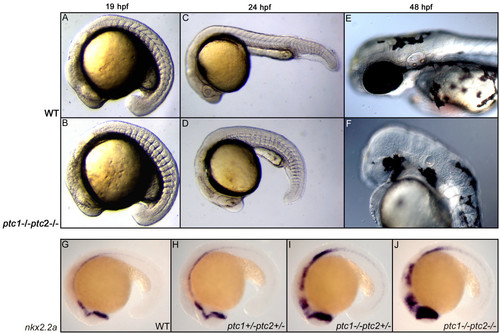
Concurrent inactivation of ptc1 and ptc2 results in severe developmental defects. At 18 hpf, a somite phenotype becomes apparent in the ptc1;ptc2 double mutants, where the chevron shaped form of the somites becomes straight (A,B), which is a typical consequence of increased activity of the Hh pathway. At 24 hpf, ptc1;ptc2 double mutants do not develop a lens but the primitive eye field is still present (C,D). At 48 hpf the eyes are completely absent. Additionally, reduced pigmentation, an absence of the nose, and an underdeveloped ear can be observed at 48 hpf (E,F). Expression levels of nkx2.2a confirms that the pathway becomes more activated upon losing wild type alleles of ptc1 or ptc2, with the highest expression in the ptc1;ptc2 mutant, mainly in the anterior brain structures (G-J). EXPRESSION / LABELING:
PHENOTYPE:
|
|
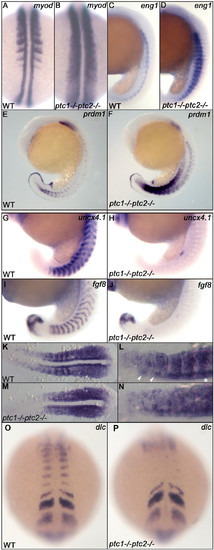
Ptc1;ptc2 double mutants show mediolateral and anteroposterior somite patterning defects. Patterning of adaxial cells and slow muscle cell precursors is disturbed in ptc1;ptc2 mutants. The region of myod positive adaxial cells and eng1 expressing slow muscle precursors are expanded at 19 hpf (A-D). At 19 hpf, prdm1 expression is highly induced in the ptc1;ptc2 mutant, suggesting that the myotome is mainly developing slow muscle type precursors (E,F). Anteroposterior patterning of the somites is lost in segmented somites, since the posterior somite marker uncx4.1 (G,H) and anterior somite marker fgf8 (I,J) are strongly reduced or not detectable at 19 hpf. myf5 expression in 11 somite stage wildtype (K,L) and ptc1;ptc2 double mutant embryos (M,N). L and N are higher magnification of relevant areas of K and M, respectively. In wild type, myf5 is expressed at higher levels in the posterior of the somites during their formation, in more posterior (younge embryos (M,N). L and N are higher magnification of relevant areas of K and M, respectively. In wild type, myf5 is exprer) segments this appears to include the adaxial cells (*). In more anterior (more mature) somites more anterior adaxial cells appear to show higher levels of labeling (arrowheads). In ptc1;ptc2 double mutant embryos (N) a "salt and pepper" type staining suggests that anterior and posterior cells are intermingled. Additionally, dlc necessary for proper segmentation is present in presomitic mesoderm but failed to be expressed in the posterior part of segmented somites (O,P), suggesting that somite formation and A/P patterning of formed somites are genetically uncoupled processes. EXPRESSION / LABELING:
PHENOTYPE:
|
|
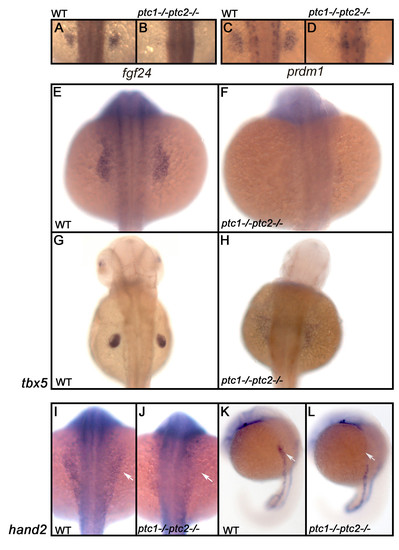
Hh signaling has an early negative role in the induction of the fin field. Expression analysis of fgf24 and prdm1 in a wild type embryo shows that these genes are restricted to the fin field at 26 hpf (A,C). ptc1;ptc2 mutants do not express these markers confirming that aberrant activation inhibits fin bud formation (B,D). To determine at which level Hh is inhibiting fin formation, the expression of tbx5, one of the earliest markers expressed in the finbud, was analyzed. At 20 hpf (E,F) tbx5 expression is lost in the presumptive finbud region (scale bar 100 μm). At 40 hpf (G,H), the fin bud has established and tbx5 expression is restricted to the pectoral fin in a wild type situation. However, in the ptc1;ptc2 double mutant a scattered low expression can be observed, showing that the pectoral fin bud is not formed. At the 10 ss, however, the initial expression domain of tbx5, encompassing heart and fin primordia is established. hand2, acting upstream of tbx5 is not expressed in the future pectoral fin area (I-L: white arrow), suggesting a very early negative role for Hh signaling in fin bud induction. EXPRESSION / LABELING:
PHENOTYPE:
|
|
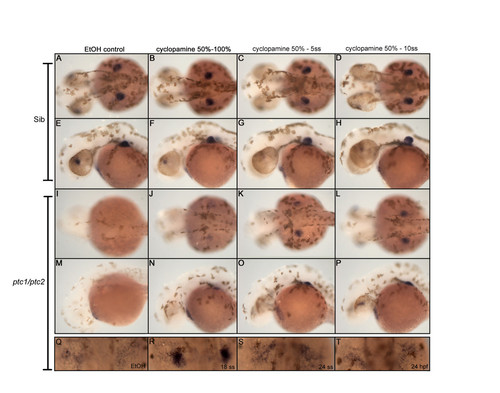
Cyclopamine treatment determines time window where Hh signaling inhibits fin induction. Treatment with 10 μM cyclopamine between 50% epiboly and the indicated developmental stages, identified the critical time window for Hh where it actively inhibits fin induction. From a dorsal (A,D, I-L) and lateral (E-H, M-P) view recruitment of tbx5 positive cells in the fin field can be slightly rescued when cyclopamine is administered between 50%- and 100% epiboly (J,N). However, tbx5 is more highly expressed when cyclopamine is administered between 50% epiboly and 5- and 10 somite stage (K,L,O,P). The constitutive activation of Hh signaling after removing cyclopamine, inhibits the outgrowth of the fin bud, clearly visible from a lateral view (M-P). Inhibiting Hh signaling in ptc1;ptc2 double mutants from the 18 ss till 40 hpf, rescues a restricted expression of tbx5, which is not, observed when cyclopamine is administered at 24 ss or 24 hpf (Q-T). These data show that Hh signaling inhibits fin induction during late gastrulation and the segmentation stage. EXPRESSION / LABELING:
|
|
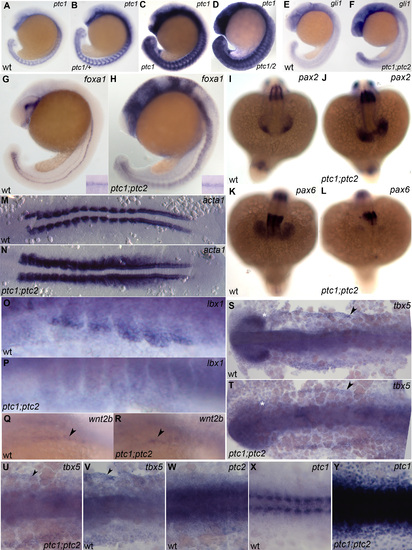
A,B,C,D ptc1 expression in wild-type (A), ptc1/+ (B), ptc1 mutant (C) and ptc1;ptc2 mutant (D) stained in a single reaction 19 somite stage; no significant increase is detectable in ptc2 mutants at this stage. E,G,I,K,M,O,Q,S,V,W,X) wild type embryos; F,H,J,L,N,P,R,T,U,Y) ptc1;ptc2 mutants. E,F) 21 somite stage; Gli expression is increased ptc1;ptc2 mutants. G,H) foxa1 expression labels medial and lateral floor plate and is expanded in ptc1;ptc2 mutants, shh a medial floor plate marker, remains unchanged (inset). I,J,K,L) 18 somite stage; pax2 labeling of the optic stalk in wild type (I) is expanded in ptc1;ptc2 mutants (J). At the same time pax6 expression in the optic cup is severly reduced in ptc1;ptc2 mutants (K,L). M,N) 12 somite stage, flat mount, dorsal view; skeletal muscle alpha actin1, a marker for muscle differentiation is increased in ptc1;ptc2 mutants. O,P) 20 somite stage, ventral part of anterior somites. Presumptive migratory myoblasts that will form fin muscle express lbx1. Expression of this gene is lost in ptc1;ptc2 mutants. Q,R) Oblique view on wnt2b expression focusing on region between lateral plate and ventral somites 21 somite stage. This gene is required for fin bud formation and is still expressed in ptc1;ptc2 mutants. S,T) 10 somite stage, flat mount, dorsal view. Initial expression of tbx5 in the -then continuous- heart and pectoral fin primordium is unaltered in ptc1;ptc2 mutants (arrowheads). Double mutants lack tbx5 expression in the optic cup (star). U,V,W,X,Y) 10 somite stage flat mount dorsal view U,V) ptc1;ptc2 mutants and wild-type show tbx5 labeling at the edge of the lateral plate mesoderm, ptc2 is also expressed in this region (W). ptc1 expression is normally only detectable in the somites strongly in the adaxial cells and weakly in the lateral somite. However, ptc1 is upregulated throughout the entire embryo in ptc1;ptc2 mutants, showing that it can respond to Hh signaling in lateral plate mesoderm. Note: X and Y were stained for equal time is the same reaction. EXPRESSION / LABELING:
|
|
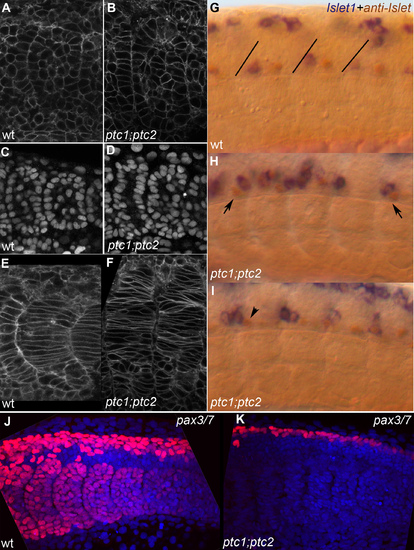
A,C,E,G,J) wild type embryo; B,D,F,H,I,K) ptc1;ptc2 mutants. A,B) lynGFP membrane label showing most recent fully formed somite, 14 somite stage. Double mutant somites still form an epithelium but irregularities in the epithelial somite are more frequent. C,D) DAPI nuclear stain showing essentially the same result as A,B. E,F) LynGFP labeling outlining cells of differentiating somites in a 14 somite wild-type and ptc1;ptc2 mutant embryo, medial optical section through somite 10 and 9+10, respectively. Somites have lost their clear V shape and the number of elongated adaxial cells appears increased. G,H,I) Double labeling showing anti-Islet (brown) and islet1 expression (20 ss). Brown cells express only Islet 2 and are Caudal Primary (CaP) neurons, blue/brown cells express islet 1 (and possibly 2) and are Middle Primary neurons (MiP). In wild-type (G) brown CaPs are located in the middle of each segment whereas the blue/brown MiPs are close to the somite boundary (drawn-in in G for clarity) [54]. In a ptc1;ptc2 mutant background (H,I) mistakes in this order are very frequent, for instance, blue and brown cells close to the posterior of the segment, or the exact mirror image of that (H, arrows). In (I) a brown cell can be seen in the position where a blue cell would be expected (arrowhead). J,K) Z-projection of Pax3/7 labeling (red) and nuclear DAPI stain (blue) on posterior 5–6 somites of 20 somite stage embryos showing the loss of all pax3/7 staining in the somites, and a reduction of the number of pax3/7 positive nuclei in the dorsal neural tube in ptc1;ptc2 double mutants. EXPRESSION / LABELING:
PHENOTYPE:
|