- Title
-
KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome
- Authors
- Lyons, D.A., Naylor, S.G., Mercurio, S., Dominguez, C., and Talbot, W.S.
- Source
- Full text @ Development
|
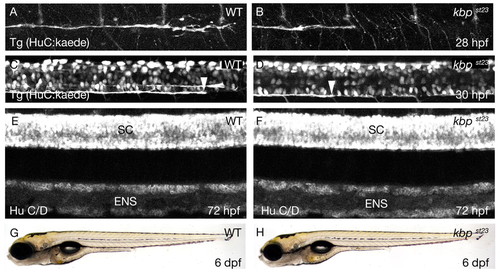
The kbpst23 mutation disrupts axonal development. (A,B) Lateral views of PLLn axons in live embryos bearing the Tg(HuC:kaede) transgene at 28 hpf. Axons of the wild type (A) have advanced further posterior than those in the kbpst23 mutant (B). The images show the segment of the nerve corresponding to about somites 6-12. Anterior is towards the left and dorsal towards the top. (C,D) Lateral views of spinal cord axons in live embryos bearing the Tg(HuC:kaede) transgene at 30 hpf. The arrowheads indicate the posterior-most axons in the ventral spinal cord tract in the wild type (C) and the kbpst23 mutant (D). The images show the segment of the spinal cord corresponding to about somites 14-20. Anterior is towards the left and dorsal towards the top. (E,F) Lateral views of embryos at 72 hpf showing neurons labeled with anti-HuC/D in the spinal cord (SC) and enteric nervous system (ENS) of a wild-type (E) and kbpst23 mutant (F). There are no discernable differences in neuronal organization between kbpst23 mutants and siblings. (G,H) Lateral views of live larvae at 6 dpf show no obvious morphological differences between wild-type (G) and kbpst23 mutant (H) larvae. The genotypes of all specimens were determined after photography (see Materials and methods). |
|
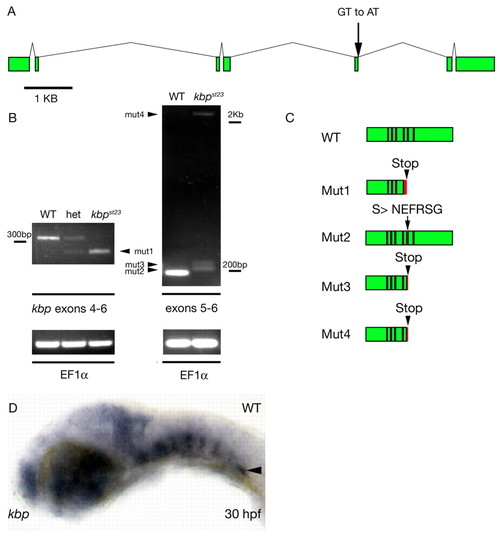
The kbpst23 mutation disrupts the zebrafish kbp gene. (A) Genomic structure of zebrafish kbp. Green boxes indicate coding sequences of kbp exons. Scale bar is 1 kb. The splice donor disrupted by the st23 lesion is indicated by the arrow. (B) RT-PCR detects aberrant kbp transcripts in kbpst23 mutant larvae. Gel on left shows RT-PCR products generated with primers from exons 4 and 6 on RNA isolated from homozygous wild-type, heterozygous and kbpst23 mutant embryos. The lower mutant band, mut1 (arrowhead), reflects the deletion of exon 5 in the spliced mutant transcript. Gel on the right shows RT-PCR products generated with primers from exons 5 and 6 from RNA isolated from a mixture of larvae with wild-type phenotypes (i.e. homozygous wild type and heterozygous) and a group of kbpst23 mutant larvae. kbpst23 mutants have three weakly expressed transcripts (arrowheads) that result from aberrant splicing at the exon 5-6 junction. Below are loading controls showing that EF1α mRNA is robustly detected in all samples. (C) Schematic depicting the structure of the wild-type KBP protein and the abnormal products predicted to result from the four mutant kbp mRNAs. Red indicates regions encoded by intronic sequence and arrowheads indicate the positions of the premature stop codons predicted to truncate the proteins encoded by mutant mRNAs. Black lines separate coding sequences derived from different exons. (D) Lateral view of a wild-type embryo at 30 hpf showing kbp mRNA expression. Anterior is towards the left and dorsal is towards the top. The arrowhead indicates kbp expression in the posterior lateral line ganglion. EXPRESSION / LABELING:
|
|
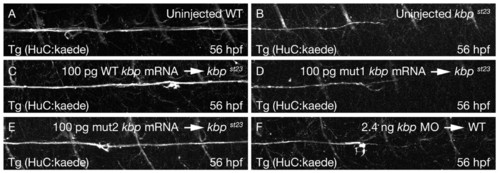
KBP is required for axonal development. Lateral views of PLLn axons in live embryos bearing the Tg(HuC:kaede) transgene at 56 hpf. The images show the segment of the nerve corresponding to about somites 10-16. Axons of the wild type (A) have advanced further posterior than those in the kbpst23 mutant (B). Axonal outgrowth is rescued in kbpst23 mutants injected with full-length wild-type kbp mRNA (C) and the mut2 kbp mRNA (E), but not in kbpst23 mutants injected with the mut1 kbp mRNA (D). Wild-type embryos injected with 2.4 ng of kbp morpholino (F) resemble kbpst23 mutants. In all images, anterior is towards the left and dorsal is towards the top. PHENOTYPE:
|
|
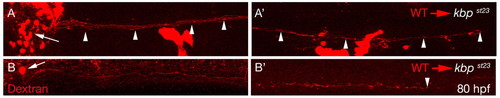
kbp is required autonomously in neurons for axonal outgrowth. (A,A′) Lateral views of a chimeric larva generated by transplantation of dextran labeled wild-type cells (red) into a kbpst23 mutant. Wild-type neurons in the PLLg (arrow, A) extend axons along the entire length of the PLL (A′). Arrowheads point to extended PLLn axons and the rightmost arrowhead in A′ represents an axon innervating the most posterior neuromast near the tip of the tail. (B,B′) Lateral views of a chimeric larva generated by transplantation of dextran-labeled wild-type cells (red) into a kbpst23 mutant. A single wild-type reticulospinal neuron in the hindbrain (arrow, B) extends an axon along the entire length of the spinal cord (B′). Arrowhead indicates the end of the axon near the tip of the tail. |
|
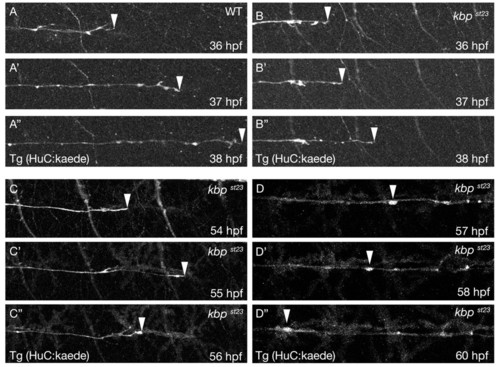
KBP is required to maintain the speed of axonal outgrowth in the PNS. (A-A″) Lateral views from a time-lapse series of a wild-type embryo with PLLn axons labeled by the Tg(HuC:kaede) transgene at 36 (A), 37 (A′) and 38 hpf (A″). Anterior is towards the left and dorsal is towards the top. The arrowheads indicate the posteriormost axons. (B-B″) Lateral views from a time-lapse series of a kbpst23 mutant embryo with PLLn axons labeled by the Tg(HuC:kaede) transgene at 36 (B), 37 (B′) and 38 hpf (B″). Anterior is towards the left and dorsal is towards the top. The arrowheads indicate the posteriormost axons. (C-C″) Lateral views from a time-lapse series of a kbpst23 mutant embryo with PLLn axons labeled by the Tg(HuC:kaede) transgene at 54 (C), 55 (C′) and 56 hpf (C″). Anterior is towards the left and dorsal is towards the top. The arrowheads indicate the posteriormost axons. (D-D″) Lateral views from a time-lapse series of a kbpst23 mutant embryo with PLLn axons labeled by the Tg(HuC:kaede) transgene at 57 (D), 58 (D′) and 60 hpf (D″). Anterior is towards the left and dorsal is towards the top. Arrowheads indicate an axonal swelling that moves backwards along the nerve. PHENOTYPE:
|
|
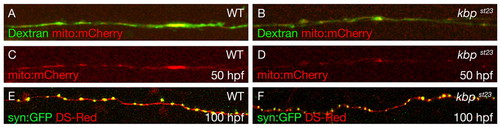
Localization of mitochondria and synaptic vesicle protein in kbpst23 mutant axons. (A-D) Lateral views of single axons at 50 hpf labeled with Oregon Green Dextran (green) and mitochondria labeled with mito:mCherry mRNA (red) in wild-type (A,C) and kbpst23 mutant (B,D) embryos. (E,F) Lateral views of single axons at 100 hpf labeled with DSRed (red) and synaptophysin labeled with syn:GFP (green) in wild-type (E) and kbpst23 mutant (F) embryos. |
|
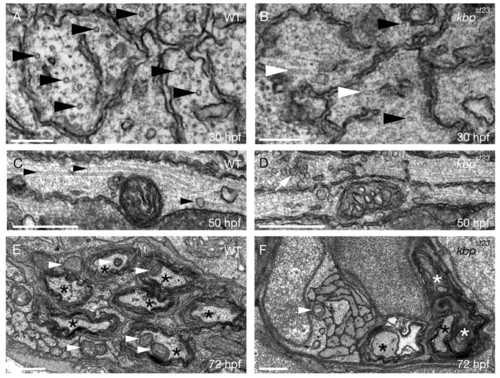
KBP is required for microtubule organization, localization of axonal mitochondria and synaptic vesicles, and axonal maintenance in the PNS. (A,B) TEM images of transverse sections through the PLLn at 30 hpf shows more organized microtubules (black arrowheads) in wild-type (A) axons than in kbpst23 mutant (B) axons and disorganized microtubules (white arrowheads) in kbpst23 mutant (B) axons. Scale bars: 200 nm. (C,D) TEM images of longitudinal sections through the PLLn at 50 hpf show more profiles of properly oriented microtubules (arrowheads) running parallel to the long axis of the axon in wild type (C) than in the kbpst23 mutant (D). White arrow indicates an accumulation of synaptic vesicles in a kbpst23 mutant axon. Scale bars: 0.5 μm. (E,F) TEM images of transverse sections through the PLLn at 72 hpf show more myelinated axons (black asterisks) and mitochondria within axons (white arrowheads) in the wild type (E) than in the kbpst23 mutant. White asterisks in F indicate myelinated axons undergoing degeneration. Scale bars: 0.5 μm. PHENOTYPE:
|
|
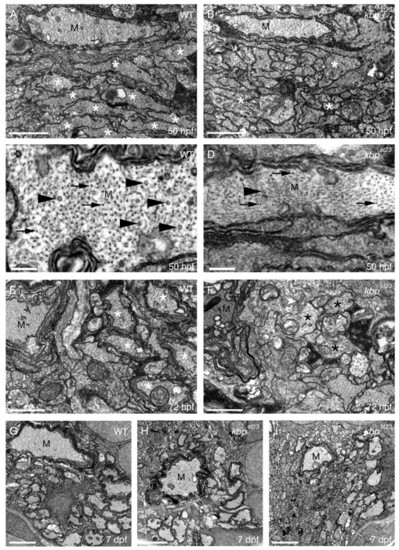
KBP is required for axonal growth, cytoskeletal organization and myelination in the CNS. (A,B) TEM images of transverse sections through the ventral spinal cord of a wild type (A) and kbpst23 mutant (B) at 50 hpf. Axons with a diameter greater than 0.75 μm are indicated by a white asterisk. There are fewer large-diameter axons in the kbpst23 mutant. Cytoskeletal disorganization is apparent in the large Mauthner axon (M) in the kbpst23 mutant compared with wild type. Scale bars: 0.75 μm. (C,D) TEM images of transverse sections through the ventral spinal cord show high magnification views of the Mauthner axon (M) in a wild type (C) and kbpst23 mutant (D) at 50 hpf. There are more properly oriented microtubules (e.g. arrowheads) and neurofilaments (e.g. arrows) in the wild type than in the kbpst23 mutant (D) Mauthner axon. Scale bars: 200 nm. (E,F) TEM images of transverse sections through the ventral spinal cord show that there are more myelinated axons (white asterisks) in wild type (E) than in the kbpst23 mutant (F) at 72 hpf. Examples of large diameter kbpst23 mutant axons with a disorganized cytoskeleton are indicated by black asterisks. Scale bars: 0.75 μm. (G-I) TEM images of transverse sections through the ventral spinal cord show that there are more large-diameter myelinated axons in the wild type (G) compared with kbpst23 mutants (H,I) at 7 dpf. Scale bars: 2 μm. |
|
KBP is required for ENS axonal development, but not for head patterning. (A,B) Lateral views of larvae at 10 dpf showing neurons labeled with anti HuC/D in the posterior hindgut of the enteric nervous system in a WT (A) and kbpst23 mutant (B). There were no discernable differences in neuronal organization between kbpst23 mutants and siblings. (C,D) TEM images of transverse sections through the enteric nervous system at 7 dpf shows a group of small axons with organized microtubules (arrowheads) in wild type (C) and more disorganized axons with accumulations of vesicles (arrow) in the mutant (D). Scale bars: 200 nm. (E,F) Ventral views of larvae at 10 dpf showing head cartilages labeled by Alcian Blue in a wild type (E) and kbpst23 mutant (F). There were no discernable differences in the organization of the head skeleton between kbpst23 mutants and siblings. |
|
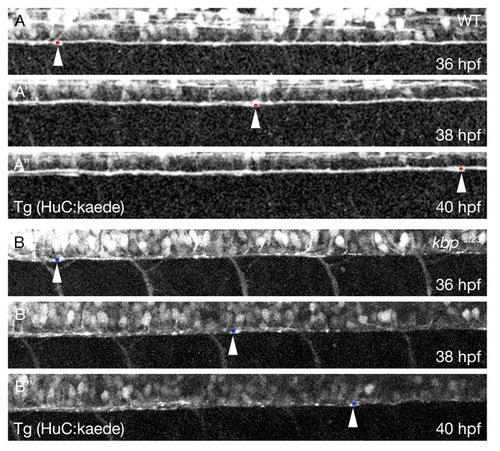
KBP regulates the speed of axonal outgrowth in the CNS. (A-A″) Lateral views from a time-lapse series of the ventral spinal cord of a wild-type embryo with axons labeled by the Tg(HuC:kaede) transgene at 36 (A), 38 (A′) and 40 hpf (A″). Anterior is towards the left and dorsal is towards the top. The arrowheads and red dots indicate the posterior-most axons. (B-B″) Lateral views from a time-lapse series of the ventral spinal cord of a kbpst23 mutant embryo with axons labeled by the Tg(HuC:kaede) transgene at 36 (B), 38 (B′) and 40 hpf (B″). Anterior is towards the left and dorsal is towards the top. The arrowheads and blue dots indicate the posteriormost axons. PHENOTYPE:
|