- Title
-
Laminin alpha5 is essential for the formation of the zebrafish fins
- Authors
- Webb, A.E., Sanderford, J., Frank, D., Talbot, W.S., Driever, W., and Kimelman, D.
- Source
- Full text @ Dev. Biol.
|
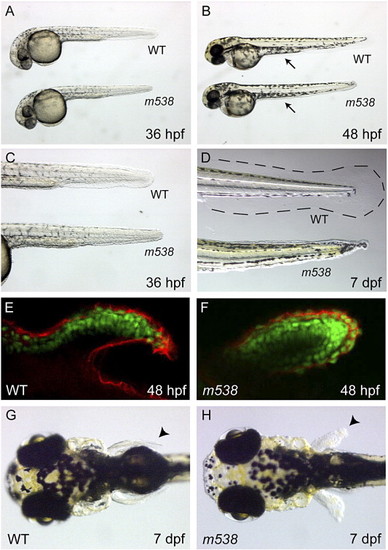
m538 mutants have severe fin and yolk extension defects. (A–D) Reductions in median fins are shown at 36 hpf (A, C), 48 hpf (B) and 7 dpf (D). In panel D, the dotted line shows the outer limit of the wild-type fin fold. Mutants often contain reduced or absent yolk extensions (B, arrows). (E–H) Anterior tissues develop normally in the mutants, with the exception of the pectoral fins. Defects in pectoral fin outgrowth can be detected at 48 hpf by immunostaining with a pan-cadherin antibody (red, E, F; green are nuclei stained with DAPI and pseudo-colored green for contrast). At 7 dpf, pectoral fins are short and stunted in m538 mutants (arrowheads in panels G and H). (A–F) Lateral views and (G, H) dorsal views; anterior view is to the left in all images. |
|
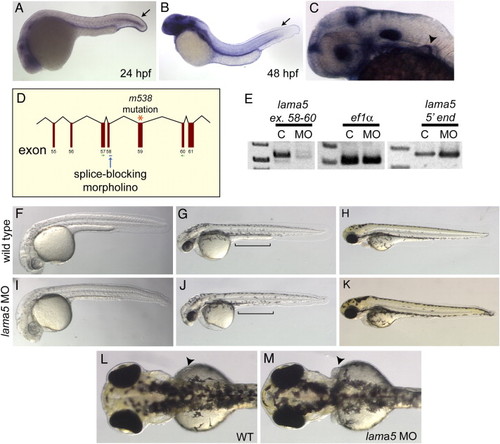
lama5 MO-injected embryos recapitulate the lama5 mutant phenotype. (A–C) By in situ hybridization, lama5 is expressed in the developing fin fold epidermis (arrows) at 24 hpf (A) and 48 hpf (B) and the developing pectoral fin (arrowhead) at 36 hpf (C). (D) The lama5 splice-blocking MO targets the exon 58 splice donor (blue arrow), one exon upstream of the m538 mutation (orange asterisk). (E) Injection of the lama5 MO successfully blocks splicing of the lama5 transcript (C, uninjected; MO, 3 ng lama5 MO; PCR primers are shown in green in panel D), but does not affect the control ef1α transcript or the 52 end of the lama5 transcript, demonstrating that the lama5 transcript is not eliminated by non-sense-mediated decay in MO-injected embryos. (F–K) Lama5 MO-injected embryos (I–K) display fin fold defects and yolk extension abnormalities (J, bracket) compared to uninjected controls (F–H) at 28 hpf (F, I), 48 hpf (G, J) and 72 hpf (H, K). (L, M) Pectoral fins at 72 hpf (arrowheads). In the morphants, the pectoral fins are abnormal (M) compared to those in wild-type embryos where the pectoral fins lie flat against the yolk (L). All images are lateral views with anterior to the left, except panels L and M, which show dorsal views with anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
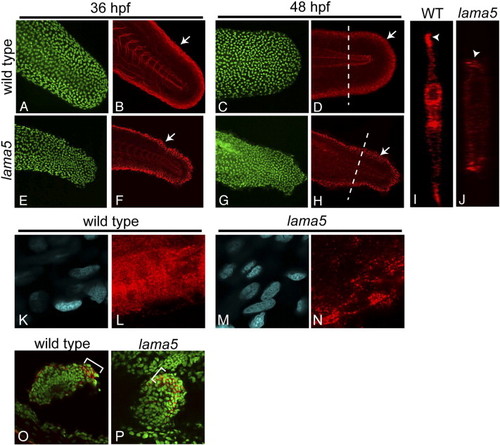
Laminin α5 is essential for basement membrane assembly in the developing fin fold. Immunostaining for laminin (red) and p63 (green) in wild-type (A–D) and lama5 mutants (E–H) shows disruptions in basement membrane along the fin fold margins (arrows) at 36 (B, F) and 48 hpf (D, H). p63 staining marks epidermal cells (A, C, E, G). (I, J) Confocal line scans (transverse sections) at the level of the dotted lines in panels D and H show laminin deposition at the tips of wild-type and mutant fin folds (arrowheads). (K–N) Higher magnification views of laminin staining in wild-type (L) and mutant (N) embryos show aberrations in basal laminae in lama5 mutants. (K, M) DAPI-stained nuclei (blue) appear morphologically normal in the affected region. (O, P) Immunostaining for laminin (red) in the pectoral fins of wild-type (O) and lama5 mutant (P) embryos at 44 hpf. Laminin proteins localize to the margin of the developing pectoral fin and are enriched in the posterior region in both wild-type and mutant embryos (brackets). DAPI-stained nuclei are pseudo-colored green for contrast. In panels A–H and K–N, dorsal is up and anterior is to the left. Pectoral fin views are dorsal with anterior to the left. |
|
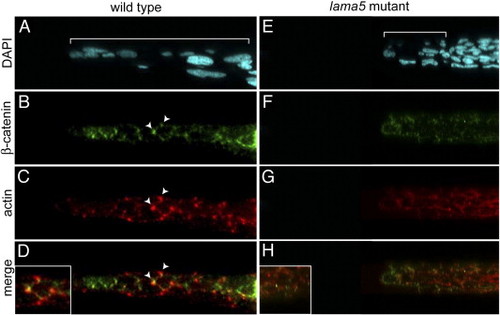
Laminin α5 is required for localization of actin and β-catenin to cell–cell boundaries. Confocal line scans (transverse sections) of the fin fold at 48 hpf in wild-type (A–D) and lama5 mutant (E–H) embryos. Nuclei are marked with DAPI (A, E) and fin folds are bracketed. Neural tube and somitic nuclei are to the right of the bracket in panel E. In wild-type embryos, β-catenin (green, B) and actin (red, C) are enriched at cell–cell boundaries (arrowheads and inset in panel D). Lama5 mutants have reduced fin fold extension (compare brackets in panels A and E) and fail to enrich β-catenin (F) and actin (G) at points of cell–cell contact (inset in H). Merged green and red channels are shown in panels D and H, dorsal is to the left. PHENOTYPE:
|
|
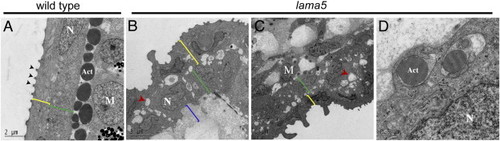
lama5 mutants have severe disruptions in epidermal organization. Transmission electron micrographs of transverse sections of the fin fold at 48 hpf in wild-type (A) and lama5 mutants (B–D). Wild-type tissue is highly organized with an outer epithelial layer (enveloping layer, yellow brackets in panels A–C) containing microridges (arrowheads) and a basal epidermal layer (green brackets in panels A–C). Large collagen bundles, termed actinotrichia (Act), run along the length of the fin fold and are shown in cross-section separating the epidermal layer from migrating mesenchymal cells (M). lama5 mutants have major defects in epidermal architecture. Cells in the inner (green brackets) and outer epidermal layers (yellow brackets) have severe morphological and adhesion defects. Large cavities appear at cell–cell boundaries (red arrowheads in panels B and C) and ECM accumulates in the sub-epidermal space (blue bracket, B). Actinotrichia are present in mutants but are reduced in size and do not localize adjacent to the basal epidermal layer. Epidermal cells can be observed wrapping around the actinotrichia (D), a cell behavior never observed in the wild-type. N, epidermal nuclei. PHENOTYPE:
|
|
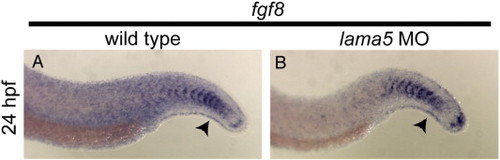
lama5 is not required for fin fold induction. In situ hybridizations for fgf8 in wild-type (A) and lama5 MO-injected embryos (B) at 24 hpf. Arrowheads indicate fin fold expression; anterior is to the left. EXPRESSION / LABELING:
|
|
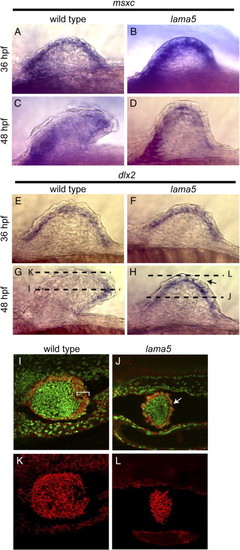
lama5 is required for formation of the apical ectodermal fold. In situ hybridizations for msxc (A–D) and dlx2 (E–H) in wild-type (A, C, E, G) and lama5 mutant (B, D, F, H) embryos. msxc and dlx2 expression appear normal in mutant embryos at 36 hpf relative to wild-type expression at this stage. Note that at 48 hpf, msxc and dlx2 are expressed in lama5 mutants, but the pectoral fins fail to elongate and form apical ectodermal folds (compare panels D and H with panels C and G). Immunostaining for cadherin (red) in wild-type (I and K) and lama5 mutant (J and L) embryos at 48 hpf marks formation of the AEF in wild-type embryos (bracket in panel I). Epidermal cells in lama5 mutants fail to form an AEF and remain as a single epithelial layer (arrow in panel J). Views of epidermal cells in wild-type (K) and mutant (L) pectoral fins show the extended morphology of wild-type epidermal cells compared to mutant epidermal cells. Anterior is to the left, panels A–H are lateral views, panels I–L are dorsal views. Dashed lines in panels G and H show the plane of the confocal sections shown in panels I–L. DAPI-stained nuclei, pseudo-colored green for contrast, are shown in panels I and J. |

Unillustrated author statements |
Reprinted from Developmental Biology, 311(2), Webb, A.E., Sanderford, J., Frank, D., Talbot, W.S., Driever, W., and Kimelman, D., Laminin alpha5 is essential for the formation of the zebrafish fins, 369-382, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.