- Title
-
The Bmp Gradient of the Zebrafish Gastrula Guides Migrating Lateral Cells by Regulating Cell-Cell Adhesion
- Authors
- von der Hardt, S., Bakkers, J., Inbal, A., Carvalho, L., Solnica-Krezel, L., Heisenberg, C.P., and Hammerschmidt, M.
- Source
- Full text @ Curr. Biol.
|
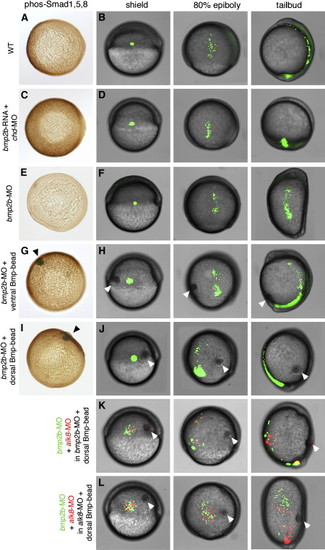
The Bmp Gradient Determines the Direction of Cell Movements during Dorsal Convergence of the Lateral Mesoderm in a Non-Cell-Autonomous Fashion (A, C, E, G, and I) Vegetal views of embryos at 85% epiboly after anti-phospho Smad1/5 immunostainings, dorsal to the right. (B, D, F, H, J–L) All other panels show live embryos after photoactivation of caged fluorescent dextran in a group of cells in the lateral mesoderm (B, D, F, H, and J) or after transplantation of a mixture of lateral mesodermal cells containing fluorescein or rhodamin dextran (K and L). Each embryo is shown at shield stage (early gastrula, 6 hpf), at 80% epiboly stage (mid-gastrula, 8 hpf), and at tailbud stage (end of gastrulation, 10 hpf). Dorsal is to the right, anterior/animal pole at the top. Embryos were injected either with bmp2b mRNA and chordin MO (C and D), with bmp2b MO (E–K), or with alk8 MO (L). To restore or generate a reverse Bmp gradient, Bmp beads (marked with arrowheads) were implanted into ventrolateral (G and H) or dorsolateral (I–L) positions of bmp2b or alk8 morphants at shield stage. PHENOTYPE:
|
|
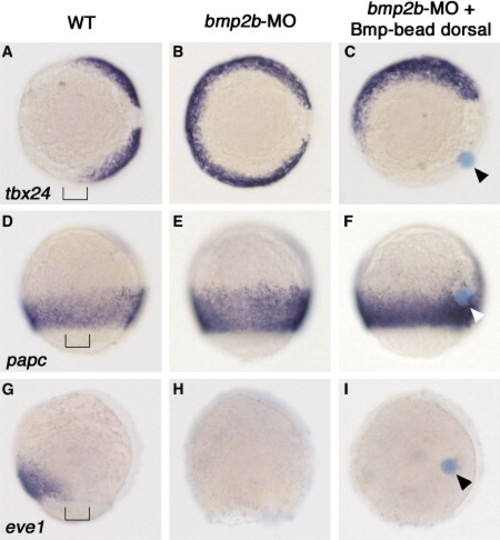
The Bmp Beads Generate a Broad Field of Laterally Specified Mesoderm. Embryos at the 80% epiboly stage, dorsal to the right, after whole-mount in situ hybridizations with probes indicated on the left side of each row, and after treatment indicated on the top of each column. Vegetal view shown in (A)–(C); lateral view shown in (D)–(I), anterior to the top. Bmp beads are indicated with arrowheads. The mesoderm throughout the entire bead-bearing side of the embryos displays absence of tbx24 and eve1, but presence of papc [46] transcripts, as is normally characteristic for a narrow lateral domain of wild-type (WT) embryos at 90° from the shield (indicated by brackets in [A], [D], and [G]). Note the presence of papc-positive cells in close proximity to the bead in (F), in line with the observation that cell movements become prominent only after mid-gastrula stages. EXPRESSION / LABELING:
|
|
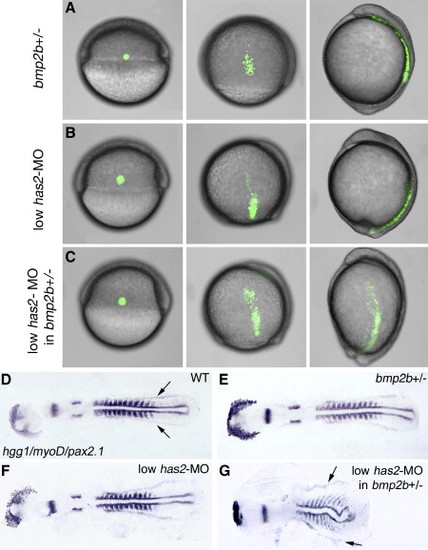
bmp2b and has2 Display Genetic Interaction during Dorsal Convergence, but Not Dorsoventral Patterning. (A–C) Live embryos with fluorescently labeled group of lateral mesodermal cells after treatment indicated on the left side of each row, at the onset, the middle, and the end of gastrulation; lateral views, dorsal to the right, anterior at the top. For wild-type controls, see Figure 1B. (D–G) Same embryos after whole-mount in situ hybridization at the 12-somite stage with hgg1, marking hatching gland precursor cells, myoD, marking muscle precursors, and pax2.1, marking midbrain-hindbrain boundary, otic placodes, and pronephric ducts. Note that in (G), the bmp2b heterozygote injected with low amounts of has2 MO displays an undulated notochord and laterally expanded somites, resulting from reduced convergence movements, while pax2.1-positive pronephric cells (indicated by arrows in [D] and [G]) are present in normal numbers, indicating that the embryo is not dorsalized (compare with [3]). EXPRESSION / LABELING:
PHENOTYPE:
|
|
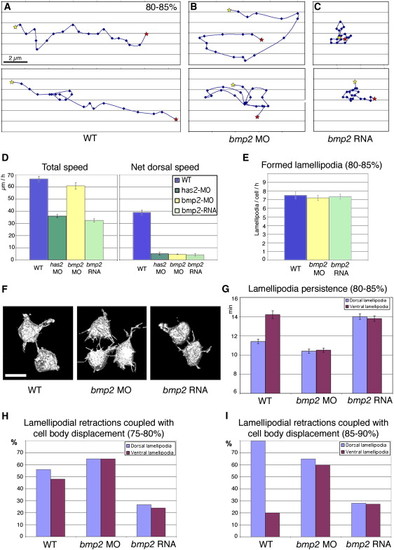
Bmp Signaling Affects the Directionality of Cell Migration and the Functionality of Lamellipodia, but Not Lamellipodia Formation Per Se (A–C) Representative examples of time-lapse recordings of individual lateral mesodermal cells over 20 min with 1 min intervals (80%–85% epiboly stage). Dorsal is to the right, animal pole to the top; the start and end points are indicated by yellow and red asterisks, respectively. (D) Graphical illustrations of the total speed and the net dorsal speed of lateral mesodermal cells (in μm/hr; n = 20) in wild-type, has2 morphant, bmp2b morphant, and bmp2b-overexpressing embryos, calculated from time-lapse recordings as shown in (A)–(C). Error bars indicate standard errors, calculated by Excel software. (E) Newly formed lamellipodia per cell and hour in wild-type, bmp2b morphant, and bmp2b-overexpressing embryos from 80%–85% epiboly. Error bars indicate standard errors, calculated by Excel software. (F) 3D confocal reconstructions of individual mGFP-labeled lateral mesodermal cells at 85% epiboly stage; dorsal to the right. All cells form multiple lamellipodia, which project into random directions. Scale bar represents 10 μm. (G) Persistence (time between outgrowth and retraction in minutes) of dorsally (in blue) and ventrally (in red) projecting lamellipodia in embryos between 80% and 85% epiboly. Error bars indicate standard errors, calculated by Excel software. (H and I) Percentages of lamellipodia of mesodermal cells at 90° from the dorsal shield, for which lamellipodial retraction was coupled with a displacement of the cell body by at least half a cell diameter into the direction of their projection. Comparison of dorsally (in blue) and ventrally projecting (in red) lamellipodia in embryos between 75% and 80% epiboly (H) and between 85% and 90% epiboly (I). For every condition/column, 20–25 lamellipodia from 4–6 independent experiments were scored. See Figure S3 for examples. WT, wild-type; MO, bmp2b morphant; RNA, bmp2b-overexpressing. The data in (D), (E), and (G) are presented as mean ± SEM. PHENOTYPE:
|
|
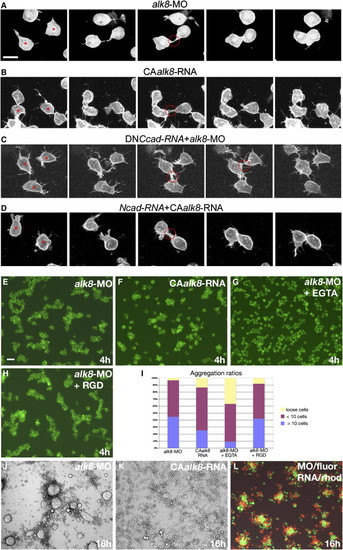
Bmp Signaling Has a Negative Effect on Cadherin-Dependent Stability of Lamellipodia-Mediated Cell-Cell Contacts In Vivo and on Cell-Cell Adhesion of Mesodermal Cells In Vitro. (A–D) Confocal time-lapse recordings of mGFP-labeled lateral mesodermal cells in embryos between 85% and 90% stage. Shown are (A) alk8 morphant cells/embryo; (B) cells/embryo injected with CAalk8-mRNA; (C) alk8 morphant cells expressing nonsecreted dominant-negative C-Cadherin after transplantation in alk8 morphant host; and (D) cells coinjected with CAalk8 and ncad mRNA after transplantation in CAalk8 mRNA-injected host. The two cells indicated with asterisks in the first photos of (A)–(D) form lamellipodia that contact each other. In (A) and (D), this contact point is stable, and cell bodies are displaced toward each other, while in (B) and (C), it is only transient, and cells remain in distance. Average direct contact durations (in min) and spatial approaches of cells during lamellipodia retractions (in μm) were: (A) >13.3 min, 9.4 ± 4.1 μm, n = 9; (B) 2.5 ± 1.6 min, 1.5 ± 0.5 μm, n = 8; (C) 5.5 ± 1.9 min, 1.3 ± 0.5 μm, n = 6; (D) >15.5 min, 11.1 ± 2.8 μm, n = 8. For (A) and (D), most cell contacts still existed at the end of the time-lapse recordings. Scale bar represents 10 μm. (E–H and J–L) Primary cultures of mesoderm progenitor cells labeled with fluorescein or rhodamin dextran and plated on fibronectin, after 4 hr (E–H) or overnight (J–L) incubation. Scale bar in (E) represents 100 μm. Embryos had been injected with alk8-MO (E, G, H, J, and L) or CAalk8-mRNA (F, K, and L). (G and H) Medium supplemented with EGTA (G) or RGD (H). Aggregation of alk8-MO cells is strongly inhibited by a reduction of extracellular Ca2+ levels but is independent of integrin-fibronectin binding. (I) Ratios of loose (yellow), small (≤10 cells; red), and large (>10 cells; blue) aggregates in alk8-MO, CAalk8-mRNA, alk8-MO + EGTA, and alk8-MO + RGD cultures after 4 hr. Cells numbers in aggregates were determined at higher magnification as described elsewhere [30]. |
|
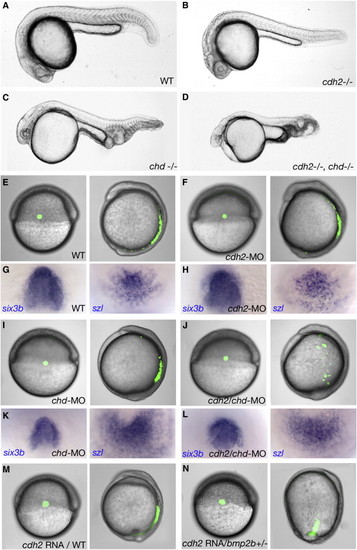
Defects in Convergence, but Not in Dorsoventral Patterning, Are Synergistically Enhanced upon Combined Loss of Chordin and N-Cadherin and in bmp2b Heterozygotes after Moderate n-cadherin Overexpression. (A–D) Lateral overviews of live embryos of indicated genotype at 24 hpf (cdh2 allele pacfr7; chd allele dintt250) (E, F, I, J, M, N) Live embryos with fluorescently labeled mesoderm cells at the onset and end of gastrulation. Coinjection of small amounts of cdh2-MO and chd-MO in wild-type embryos (J) or injection of small amounts of cdh2-mRNA in bmp2b+/- embryos (N) lead to a synergistic enhancement of the defects in convergence movements. (G, H, K, L) Same embryos as shown in (E), (F), (I), (J), at 5-somite stage and after in situ hybridizations for six3b (left, animal views), a marker for anterior neuroectoderm, and szl (right, vegetal views), a marker for posterior mesoderm. The chd/cdh2 double morphant (L) displays a six3b expression domain not smaller than that of the chd single morphant (K), and a szl domain not larger than that of the chd morphant, indicating that the ventralization caused by loss of Chd is not further enhanced by the simultaneous loss of Cdh2. EXPRESSION / LABELING:
PHENOTYPE:
|
|
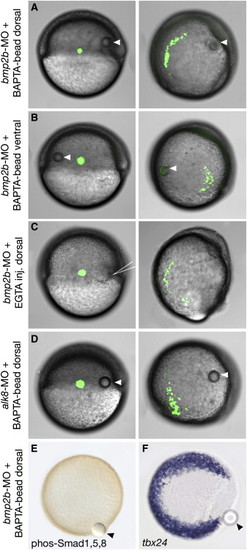
Locally Applied Ca2+ Chelators Induce Movement of Lateral Mesodermal Cells in an Alk8-Independent Fashion and without Affecting Dorsoventral Patterning (A)–(D) Live embryos with a fluorescently labeled group of lateral mesodermal cells at the onset of gastrulation (shield) and at a late gastrula stage (90% epiboly). (A, E, and F) bmp2b morphants with dorsolateral BAPTA beads. (B) bmp2b morphant with ventrolateral BAPTA bead. (C) bmp2b morphant after dorsolateral injection of EGTA (arrow). (D) alk8 morphant with dorsolateral BAPTA bead. (E and F) Vegetal views of embryos at 80% epiboly stage; (E) anti-phos Smad1/5/8 immunostaining; (F) tbx24 in situ hybridization. Implanted beads are indicated by arrowheads. |
|
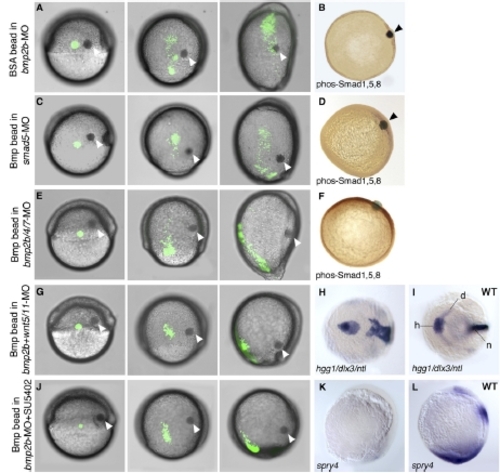
The Movement-Inducing Effect of the Bmp Beads Requires Smad5, but Is Independent of Endogenous Bmp2b/4/7 and of Noncanonical Wnt and Fgf Signaling. (A, C, E, G, and J) Live embryos treated as indicated on the left side of each row, at three different stages of gastrulation: shield stage (column 1), directly after bead implantation and subsequent fluorescein-uncaging of a group of lateral mesodermal cells; at 80% epiboly stage (column 2); and tailbud stage (column 3). Dorsal is to the right, anterior to the top. Implanted BSA beads (A, B) and Bmp beads (other panels) are indicated by arrowheads. (B, D, and F) Anti-phosphorylated Smad1/5/8 immunostainings at 80% epiboly, vegetal view, dorsal to the right. (A and B) Lateral mesodermal cells of bmp2b morphants do not respond to control BSA beads. (C–F) smad5 morphants do not respond to Bmp beads (C and D), whereas bmp2b/bmp4/bmp7 triple morphants do (E and F), indicating that the effect of exogenous Bmp requires transcriptional activation of Bmp target genes but is independent of the transcriptional positive feedback loop of Bmp signaling. (G–L) bmp2b morphants respond to the Bmp beads even after coinjection of wnt5 and wnt11 MOs (G) and after treatment with SU5402 (J), indicating that the effect is independent of noncanonical Wnt and Fgf signaling. (H and I) A bmp2b/wnt5/wnt11 triple morphant sibling (H) and a wild-type control (WT; [I]) not implanted with beads, after whole-mount in situ hybridization for the hatching gland marker hgg1 (h), the neural crest marker dlx3 (d), and the notochord marker ntl (n); tailbud stage, animal view, dorsal to the right. The triple morphant lacks dlx3 expression, resulting from the dorsalization caused by loss of Bmp2b, and strongly impaired convergent extension movements, caused by the loss of Wnt5 and Wnt11, as reflected by the broader notochord and the reduced anteriorposterior distance between hatching gland anlage and notochord. (K and L) bmp2b morphant treated with SU5402 (K) and wild-type control (WT; [L]) after in situ hybridization for sprouty4; lateral view, dorsal to the right, anterior at the top. The SU5402-treated bmp2b morphant lacks sprouty4 expression, indicating loss of Fgf signaling. |
|
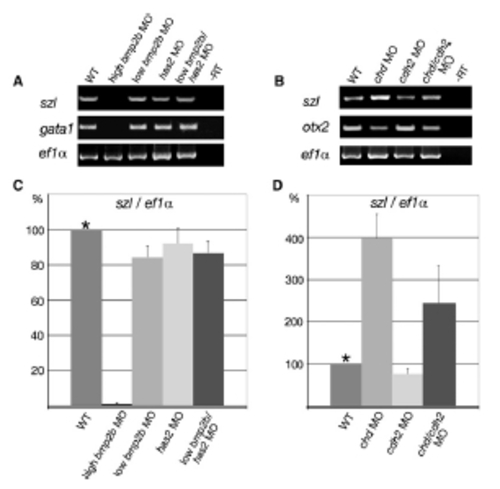
RT-PCR Analyses of Embryos Coinjected with Low Amounts of bmp2b and has2 MOs, or Coinjected with chd and cdh2 MOs, Demonstrating that Dorsoventral Patterning Is Not More Perturbed than in the Respective Single Morphants (A) Semiquantitative RT-PCR with total RNA preparations from embryos at tailbud stage (10 hpf), amplifying transcripts of the ventral marker gene szl [S7, S8] (24 cycles), or from embryos at the 10-somite stage, amplifying transcripts of the blood precursor gene gata1 [S9] (26 cycles). For both stages, transcripts of the translation elongation factor gene ef1α [S10] were amplified as control (20 cycles). Lane 1, uninjected control; lane 2, injection of 0.1 mM bmp2b MO; lane 3, injection of 0.01 mM bmp2b MO; lane 4, injection of 0.1 mM has2 MO; lane 5, coinjection of 0.01 mM bmp2b MO and 0.1 mM has2 MO. In bmp2b, has2 double hypomorphants, szl and gata1 transcript levels are not lower than in bmp2b single hypomorphants, indicating that they are not more strongly dorsalized, in contrast to their enhanced convergence defects (compare with Figure 3). (B) Semiquantitative RT-PCR with total RNA preparations from embryos at the tailbud epiboly stage (10 hpf), amplifying transcripts of the ventral marker gene szl [S7, S8] (24 cycles), the dorsal marker gene otx2 [S6] (25 cycles), or ef1α (20 cycles). Lane 1, uninjected control; lane 2, injection of 0.05 mM chordin MO; lane 3, injection of 0.1 mM cdh2 MO; lane 4, coinjection of 0.05 mM chordin MO and 0.1 mM cdh2 MO. In chd, cdh2 double morphant, szl transcript levels are not higher, and otx2 transcript levels not lower than in chd single morphants, indicating that the double morphants are not more strongly ventralized, in contrast to their enhanced convergence defects (compare with Figure 6). (C and D) Real-time RT-PCR of samples at tailbud stages shown in (A) or (B), respectively, amplifying transcripts of szl and ef1α. Normalized szl versus ef1α values of uninjected wild-type control embryos (WT) are set to 100%. Graphs summarize results from four different PCR reactions (duplicates of two independent experiments). Error bars indicate standard errors, calculated by LightCycler Software 3.5 (Roche). Siblings of embryos processed for RT-PCR were analyzed for convergence defects and displayed the same phenotypes as shown in Figures 3 and 6. EXPRESSION / LABELING:
|