- Title
-
Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish
- Authors
- Hammond, C.L., Hinits, Y., Osborn, D.P., Minchin, J.E., Tettamanti, G., and Hughes, S.M.
- Source
- Full text @ Dev. Biol.
|
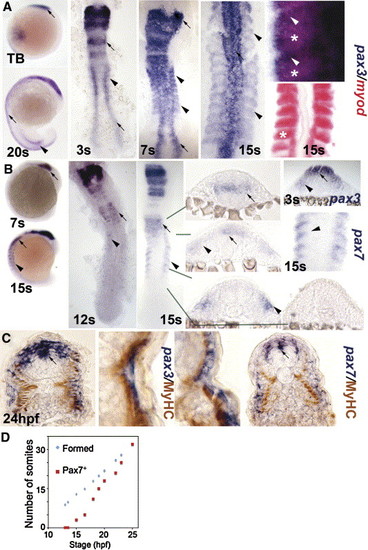
Pax3 and pax7 expression becomes restricted to the surface of zebrafish somites. In situ mRNA hybridisation for pax3 (A, C left, blue), pax7 (B, C right) and myod (A, red) or immunodetection of slow muscle (C, brown). Whole mounts (A, B) are lateral views, dorsal to left. Flat mounts (A, B) are dorsal view, anterior to top. Transverse cryosections of wholemount-stained embryos (B, C) have dorsal to top. (A, B) Pax3 and pax7 mRNA in neural tissue (arrows) and somites (arrowheads). Wholemount embryos show how pax3 is broadly expressed in new somites, whereas pax7 increases after pax3 mRNA becomes restricted to the anterolateral somite. In high magnification views of somites, note the distinct location of myod in posterior somite (asterisks) and pax3 in anterior (arrowheads). (C) Pax3 and pax7 expression is superficial to slow myosin. Low magnification caudal somite; high magnification rostral somite. (D) Plot of number of somites with pax7 mRNA signal in embryos of each developmental stage. Expression is always in a single series of rostral somites. Note the lag in pax7 expression compared to somite formation. EXPRESSION / LABELING:
|
|
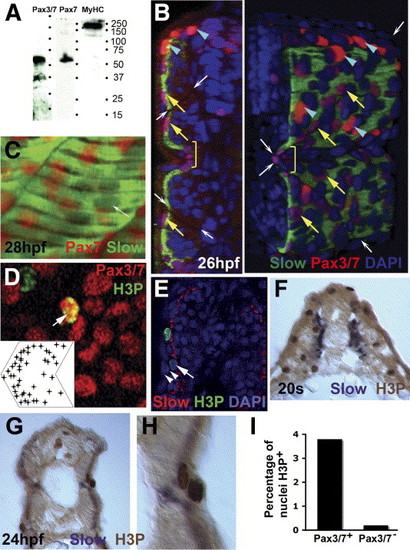
Pax3/7 protein accumulates in nuclei on the somite surface. Immunodetection of slow muscle (B, C, green; E, red; F-H, purple), Pax7 (C, red), Pax3/7 (B, D, red) or H3P (D, E, green; F-H, brown). Wholemount 24 hpf confocal images (B-E) in transverse reconstruction (B, E left), lateral view (C, D, anterior to left) or rendered 3D reconstruction (E right) or transverse cryosection at 20 som (F) or 24 hpf (G, H) are shown with dorsal to top. (A) Western blot of 24 hpf embryo proteins reveals a similar ∼ 50 kD band with both Pax7 and Pax3/7 antibodies. MyHC shows the specificity of the reagents. (B) Confocal images reveal two types of Pax3/7-expressing nuclei (red/purple). Left panel, transverse section of a yolk extension level somite showing superficial location of Pax-expressing cells. Right panel, rendered ¾ view showing infrequent intensely-labelled red nuclei located dorsal to the neural tube and lateral to the dorsal somite (blue arrowheads). More abundant weakly-labelled purple nuclei are evenly spread over the lateral somite surface (yellow arrows), but tend to congregate near somite borders (indicated by white arrows) and at the horizontal myoseptum (yellow bracket). Note weak Pax3/7 immunoreactivity in nuclei of dorsal neural tube, floor plate and between somite and neural tube. The latter may be medially-migrating neural crest. (C) A short confocal stack showing unlabelled slow muscle fibre nuclei (white arrow) and Pax7 immunoreactivity (red). (D) Pax3/7 (red) and phosphohistone H3 (green) dual stained nuclei (arrows). Inset plots distribution of Pax3/7 and H3P dual labelled nuclei on a schematised somite from analysis of somites 12 and 13 in 20 wild type fish. Nuclei with only H3P are generally in the epidermis. (E) The non-epidermal nature of an H3P-labelled nucleus (arrow) superficial to the slow fibre layer is demonstrated by the two layers of overlying epidermal cell nuclei (arrowheads). (F-I) At 20 s stage, abundant H3P is present in the lateral somite, which consists chiefly of undifferentiated fast muscle precursors (F). After fast fibre formation and slow fibre migration, H3P is generally restricted to the somite surface lateral to slow myosin (G). Note the presence of two dividing cells in adjacent cell layers, confirming that somitic external cells divide (H). Quantification of the fraction of DAPI-labelled nuclei in 80 somites 12-16 of 24 hpf embryos that express H3P in Pax3/7+ or Pax3/7- cells. Total H3P+ nuclei counted were 114 and 38, respectively. Somites in this region and stage contain 271 nuclei ± 19 sem, n = 4, estimated from confocal stacks (I). EXPRESSION / LABELING:
|
|
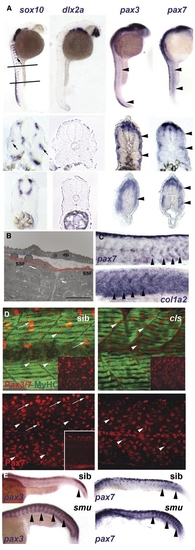
Neural crest does not account for superficial somite pax3/7 expression. (A) In situ mRNA hybridisation at 27 hpf for sox10, dlx2a, pax3 and pax7. Upper panels show lateral views, dorsal to left anterior to top, lines indicate locations of cryosections in lower panels. Note the punctate distribution of sox10 expression (arrows) on lateral pathway only in anterior sections compared with uniform distribution of pax3/7 mRNA at all axial levels (arrowheads). (B) Electron micrograph of longitudinal section of 24 hpf somite revealing rounded cells (blue) overlying flattened external cells (red) superficial to myofibril-containing slow fibres (SSF). ep, epidermis. Scale bar = 10 μm. (C) In situ hybridisation of 27 hpf embryos reveal the increase of col1α2 as pax7 declines (arrowheads). (D) Lateral view, dorsal to top anterior to left of confocal stacks of superficial region of wholemount immunodetection for Pax3/7 (red, upper panels) or Pax7 (red, lower panels) and myosin (MF20, green) of 24 hpf sibling (left) and colourless (right) embryos. Note the strong Pax-expressing cells (putative neural crest cells, arrows) are missing in cls, whereas the abundant weak Pax-expressing cells are still present (arrowheads). Insets show Pax channel alone at superficial (upper insets) or deep (lower inset) level within the somite. (E) Lateral views of wholemount in situ mRNA hybridisation for pax3 and pax7 in smoothened mutants and control siblings at 24 hpf. Note the persistence of abundant mRNA in the somitic region as somites mature in the absence of slow muscle (arrowheads). EXPRESSION / LABELING:
|
|
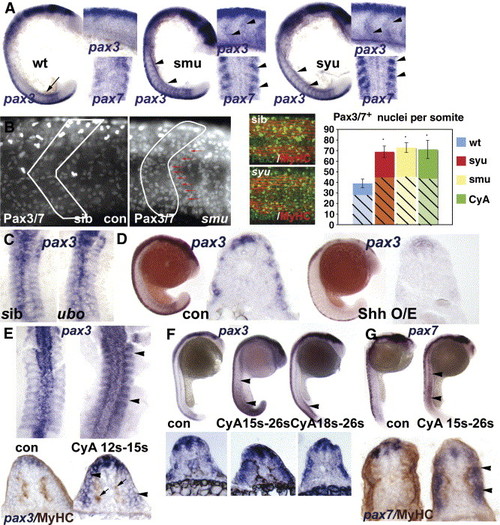
Hedgehog signalling positively regulates somitic pax3/7 gene expression. (A) Flatmounts of wholemount in situ mRNA hybridisation for pax3 (at 18 s main panel, dorsal to left; upper inset, dorsal to top) and pax7 (at 15 s lower inset dorsal view of somites 3–6) in wild type, smu and syu mutant embryos. Note the transient expression of high level pax3 in nascent somites in wild type (arrow), and its persistence in anterior somites of mutants, particularly at the somite borders (arrowheads). (B) Confocal immunodetection of Pax3/7 protein increase in somites of 24 hpf smu (left panels, somite border outlined) and syu (central panels) embryos. Note the clustering of Pax-expressing cells near the anterior somite border (red arrows). Graph shows quantification of number of nuclei labelled with Pax3/7 (plain strong colour) or Pax7 (hatched pastel shade) in each condition from somites 12–16. Note that the Pax7 antibody gives less signal and more background, which probably explains the lower counts. Counts of three somites in 4–7 animals, error bars show standard deviation. Asterisks: P < 0.001. (C) Pax3 expression is indistinguishable in dorsal flatmounts of 15 s u-boot and sibling embryos. (D) Sonic hedgehog mRNA injection into the early embryo decreases pax3 mRNA at 18 s. Left panels wholemount, dorsal to left. Right panels, wholemount cryosection at yolk extension level. (E-G) Transient exposure to cyclopamine at the indicated stages leads to localised up-regulation of somitic pax3 (E, F) and pax7 (G). Upper panels wholemounts, dorsal to left. Lower panels, wholemount cryosection, dorsal to top. (E) Dorsal flatmount showing that treatment from 12 s to 15 s up-regulates pax3 in all somites (arrowheads) without affecting slow muscle formation (arrows, lower panels). (F) Treatment from 15 s up-regulates pax3 along most of rostrocaudal axis at 26 s (arrowheads), whereas treatment from 18 s has little effect in anterior somites, shown in transverse section below. (G) Cyclopamine from 15 s up-regulates pax7 throughout the axis at 26 s, but only in somitic tissue superficial to the slow muscle (arrowheads). EXPRESSION / LABELING:
|
|
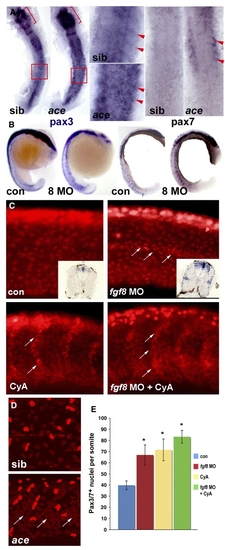
Fgf8 signalling negatively regulates pax3/7 expression. In situ mRNA hybridisation for pax3, pax7 or immunodetection of Pax3/7 in ace mutants or embryos injected with fgf8 MO or vehicle, followed by treatment with cyclopamine or vehicle and analyzed at 15 s (A, dorsal flatmount anterior to top), 18 s (B, lateral wholemount dorsal to left), 24 hpf (C) or 26 hpf (D, lateral confocal stacks of midbody somites, dorsal to top, anterior to left). (A) Both pax3 and pax7 mRNA are up-regulated in ace mutants, which were identified by their midhindbrain pattern defects (brackets). Note pax3/7 increase in the anterior of somites (arrowheads, boxes are enlarged), relative to neural tube. (B) Pax3 and pax7 up-regulation in all somites after fgf8 MO injection. (C) Pax3/7-containing nuclei are increased in number by fgf8 MO, cyclopamine or both. Fgf8 MO particularly increases staining at the horizontal myoseptum, in contrast to cyclopamine which increases cells on the somite borders (arrows). Note the lack of change in intensity of immunofluorescence and the lack of significant neural crest migration in both control and treated posterior somites. Insets show pax3 mRNA up-regulation persisting to 33 hpf. (D) Ace mutant embryos (identified by cerebellar morphology) have more weak Pax3/7 immunoreactive nuclei than siblings. Note clustering around the horizontal myoseptum (arrows) and more extensive neural crest migration in these older embryos compared to panel C. (E) Quantification of Pax3/7-expressing cells per somite after treatments indicated. Data represent mean ± standard deviation of counts from four somites in at least 4–7 embryos. Asterisks indicate significant difference from control, P < 0.001. EXPRESSION / LABELING:
|
|
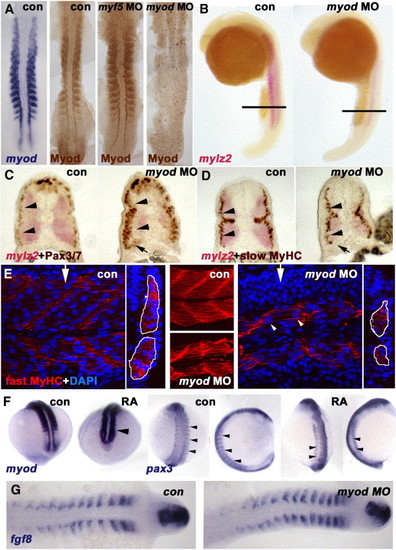
Myod is required for normal fast muscle differentiation and suppression of Pax3/7. In situ mRNA hybridisation for myod, mylz2 myosin light chain, fgf8 or pax3 or immunodetection of Myod, Pax3/7, slow or fast MyHC viewed in dorsal flatmount (A, G 15 s), lateral wholemount (B, 26 s dorsal to right), transverse section of wholemount (C, D, 26 s dorsal to top), confocal stack (E, middle) or section in lateral view (E, 24 hpf medial somite anterior to left, dorsal at top, with transverse projection at white arrows shown to right) and wholemount (F, mid somitogenesis, anterior to top). (A) Myod-specific immunoreaction co-localises with myod mRNA. Injection of myod morpholino oligonucleotide (MO) ablates Myod protein, whereas myf5 MO enhances Myod immunoreaction. (B-D) Myod MO reduces mylz2 mRNA in fast muscle (B-D), without affecting slow muscle differentiation (D). Cryosections at the level indicated in panel B reveal reduced lateral migration of slow fibres (D), correlating with increased lateral Pax3/7-expressing cells (C). Pax3/7 expression in neural tube/crest appears unaffected. Note that all fast fibres appear medial to slow fibres, that most Pax3/7+ cells lie lateral to slow fibres (arrowheads C, D), but that Pax3/7+ cells accumulate ventrally next to the residual fast muscle (arrows C, D). (E) Similarly, myod MO reduces the quantity of muscle labelled for fast MyHC (outlined in white in transverse projections). Note that some fibres still appear multinucleate (arrowheads). (F) Exposure of embryos to 10- 7 M retinoic acid up-regulates myod mRNA in lateral presomitic mesoderm and somite (arrowhead, left pair, 15 s dorsal view of tailbud), RA reduces pax3 mRNA in nascent somites of 13 s embryos (arrowheads, shown at left in dorsal view and to right in lateral view). Note the up-regulation of pax3 signal in neural tissue. (G) Myod MO has no effect on fgf8 mRNA accumulation. EXPRESSION / LABELING:
|
|
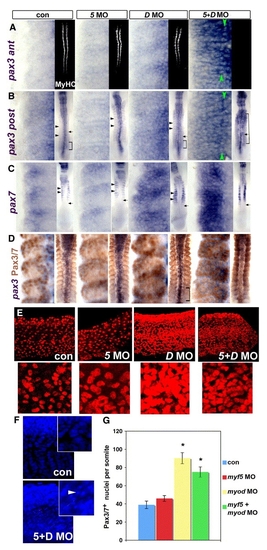
MRFs act to suppress Pax3/7 expression during somite development. Immunodetection of MyHC or Pax3/7 or in situ mRNA hybridisation for pax3 or pax7 in embryos injected with MRF MOs viewed in dorsal flatmount (A-D 15 s anterior to top), lateral confocal short stack of yolk extension somites at 24 hpf (E, magnified in insets) and confocal stack in lateral view (F, 24 hpf, DAPI). (A insets) Injection of both myf5 and myod MOs ablates muscle, whereas each MO alone has no effect on MyHC. (A-C) Myod MO or double MO up-regulate pax3 both in anterior (som 8-10, A) and posterior (som 11-13, B) somites and pax7 (C) mRNA. Green arrowheads indicate adaxial cell rows. Insets show the normal timing of pax7 accumulation after pax3 (arrows). Brackets show somites in which pax3 is widely expressed. Black arrowheads indicate posterior region of more mature somites in which pax3/7 mRNA is low relative to the anterior region. Myf5 MO is like controls. (D) Dual immunodetection of pax3 mRNA and Pax3/7 protein. (E) Pax3/7 protein is up-regulated in many lateral somite cells by either myod MO alone or double MO, but is little affected by myf5 MO. Insets show a single region at higher magnification. (F) DAPI reveals numerous somite nuclei with condensed chromatin (arrowhead), suggesting apoptosis in double MO embryos. Note that the apparent reduction in tissue mass may lead to an underestimate of the extent of the Pax3/7 increase. (G) Counts at 24 hpf represent mean ± standard deviation of four somites in 4–6 embryos. Asterisks indicate significant difference from control, P < 0.001. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 302(2), Hammond, C.L., Hinits, Y., Osborn, D.P., Minchin, J.E., Tettamanti, G., and Hughes, S.M., Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish, 504-521, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.