- Title
-
Zebrafish Staufen1 and Staufen2 are required for the survival and migration of primordial germ cells
- Authors
- Ramasamy, S., Wang, H., Quach, H.N., and Sampath, K.
- Source
- Full text @ Dev. Biol.
|
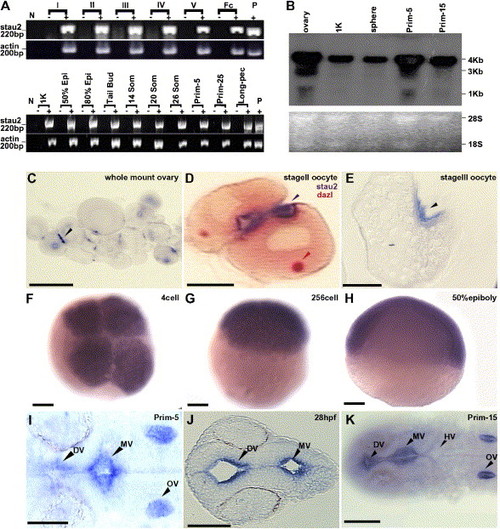
Expression of stau2 in oocytes and embryos. (A) RT-PCR analysis of stau2 expression in comparison to actin in oocytes (top panel) and embryos (bottom panel) (stages indicated above lanes). Abbreviations: fc, follicle cells; epi, epiboly; som, somites; N, no template control; P, plasmid control; − indicates RT-control for each stage. (B) Northern Blot analysis of stau2 expression. A predominant 4 kb transcript is detected in oocytes and embryos. In addition, a shorter 3 kb transcript is seen in oocytes and prim-5 embryos. Lower panel, Ethidium bromide stained 28S and 18S rRNA loading controls. (C–K) Spatiotemporal expression of stau2 by whole mount (C, F–I, K) or section (D, E, J) in situ hybridizations on oocytes (C–E) and embryos (F–K); black arrowheads, stau2; red arrowhead, dazl; stau2 is expressed in 2–3 cortical patches in oocytes (C–E), is uniform in early cleavage stage embryos (F–H), and is localized in embryos from late segmentation stages (I–K). Abbreviations: DV, diencephalic ventricle; MV, midbrain ventricle; HV, hindbrain ventricle; OV, otic vesicle. Scale bars, 100 μm. |
|
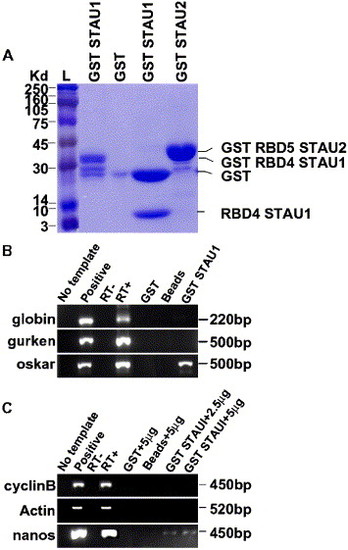
GST-Stau1 fusion protein binds to oskar and nanos1 RNA. (A) SDS-PAGE analysis of purified GST fusion proteins. L, Rainbow ladder, GST Stau1 RBD4, GST, GST Stau1 RBD4 digested with thrombin protease to release GST (28 kDa) and Stau1 RBD4 (7 kDa), GST Stau2 RBD5. (B) RT-PCR of globin, gurken, or oskar cDNAs from GST Stau1 RBD4 beads. No template control, positive globin, gurken, or oskar plasmid controls, RT−, RT+, GST (1 μg) incubated with 250 ng of globin, gurken, or oskar RNA, Control Glutathione beads incubated with 250 ng of globin, gurken, or oskar RNA, and GST Stau1(1 μg) beads incubated with 250 ng of globin, gurken, or oskar RNA. (C) RT-PCR from GST Stau1 RBD4 beads incubated with zebrafish ovary RNA. No template control, positive cyclinB, actin, or nanos plasmid controls, RT−, RT+, GST protein (1 μg) incubated with 5 μg of zebrafish ovary RNA, Glutathione beads incubated with 5 μg of zebrafish ovary RNA, GST Stau1 beads (1 μg) incubated with 2.5 μg of zebrafish ovary RNA, and GST Stau1 beads (1 μg) incubated with 5 μg of zebrafish ovary RNA. |
|
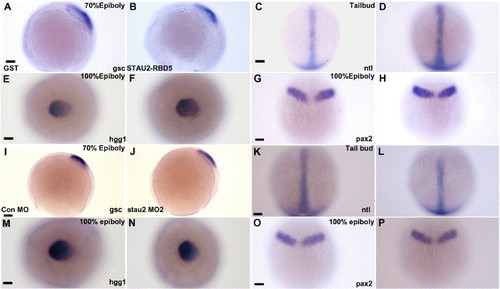
Germ layer patterning is not affected by disruption of Stau2 function. Expression of gsc (A, B, I, J), hgg (E, F, M, N), ntl (C, D, K, L), and pax2 (G, H, O, P) in embryos injected with GST (A, E, C, G), Stau2 RBD5 (B, F, D, H), Control morpholino (I, M, K, O), or stau2 morpholino2 (J, N, L, P); Dorsal views with the exception of panels A, B, I, and J (lateral views). Scale bars, 100 μm. |
|
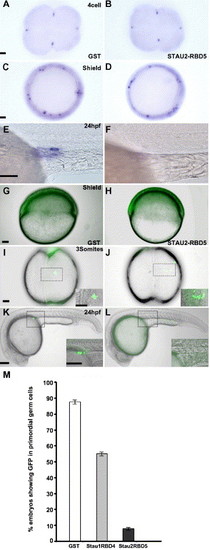
Stau1 and Stau2 peptides overexpression abolishes PGCs. (A–F) Expression of the germ cell specific gene, vasa, in 4 cell (A, B), shield stage (C, D), and 24 hpf (E, F) embryos injected with GST (A, C, E), Stau2 RBD5 (B, D, F); GFP:nos1 expression in shield stage (G, H), 3-somite stage (I, J), and 24 hpf (K, L) embryos injected with GST (G, I, K), Stau2 RBD5 (H, J, L), histogram in M shows % embryos with GFP expression in PGCs. In panels I–L, dotted boxes outline the areas shown at higher magnification in the insets. A–D, animal pole views; E–H and K, L, lateral views; I, J dorsal views. Scale bars, 50 μm for panels A, C, G, I and 100 μm for panels E, K, and insets. |
|
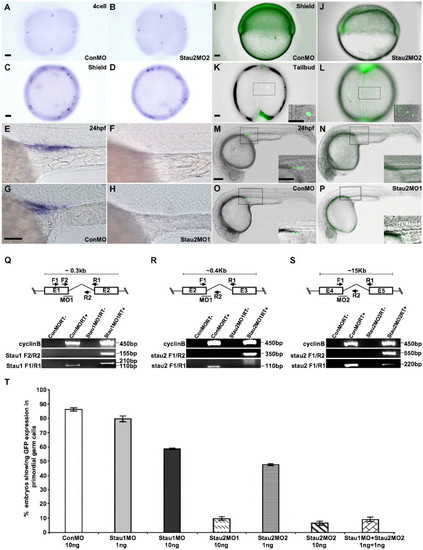
Injection of stau1 and stau2 morpholinos causes loss of germ cells. (A–H) Expression of the germ cell specific gene, vasa, in 4 cell (A, B), shield stage (C, D), and 24 hpf (E–H) embryos injected with control morpholinos (A, C, E, G), Stau2MO1 (H), or Stau2 MO2 (B, D, F). GFP:nos1 expression in shield stage (I, J), 3-somite stage (K, L) and 24 hpf (M–P) embryos injected with control morpholinos (I–O), Stau2 MO1 (P), or Stau2 MO2 (J–N). Schematic diagrams in panels Q, R, and S show the position of morpholinos targeting exon junctions in stau1 (Q) or stau2 (R, S), with primer positions indicated. RT-PCRs show amplification of cyclinB control cDNA, stau1 (Q) or stau2 (R, S) products in control or morpholino-injected embryos. Histogram in panel T shows % embryos with GFP expression in PGCs. Scale bars, 50 μm in panels A, C, I, K and 100 μm in panels G, M, and insets. |
|
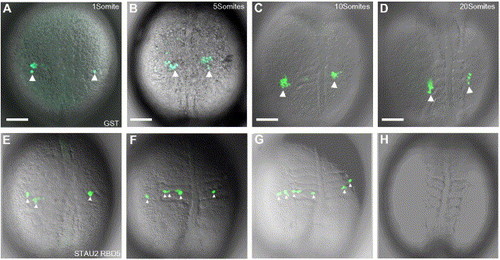
PGC migration is aberrant when Stau2 function is disrupted. Time-lapse microscopy on GST-injected embryos (A–D) shows clusters of GFP:nos expressing cells (large white arrowheads) that migrate to the gonadal ridge. In contrast, in embryos injected with Stau2 RBD5 (E–H), GFP expressing cells fail to form clusters, remain dispersed (small white arrowheads in panels E, F, G), do not migrate to the gonadal ridge, and eventually disappear (H). Dorsal views of 1 somite stage (A, E), 5 somite stage (B, F), 10 somite stage (C, G), and 20 somite stage (D, H) embryos. Scale bars, 100 μm. |
|
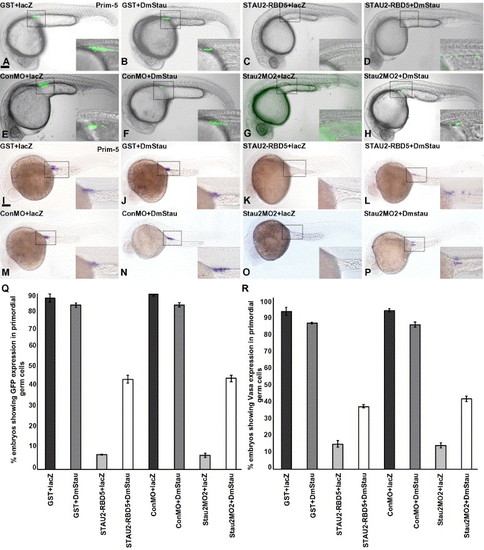
Loss of PGCs in Stau disrupted embryos is rescued by co-injection of Drosophila staufen. GFPnos1 expression (A–H, Q) or vasa expression (I–P, R) in embryos (24 hpf) injected with GST protein + lacZ RNA (A, I), GST protein + Drosophila melanogaster staufen RNA (B, J), Stau2 RBD5 peptide + lacZ RNA (C, K), Stau2 RBD5 peptide + Drosophila melanogaster staufen RNA (D, L), control morpholinos + lacZ RNA (E, M), control morpholino + Drosophila melanogaster staufen RNA (F, N), Staufen2 morpholino2 + lacZ RNA (G, O), and staufen2 morpholino2 + Drosophila melanogaster staufen RNA (H, P). Dotted boxes in panels A–P outline the areas shown at higher magnification in the insets. Lateral views, scale bar, 100 μm. (Q) % embryos with GFPnos expression in PGCs, (R) % embryos with vasa RNA expression at 24 hpf. |
|
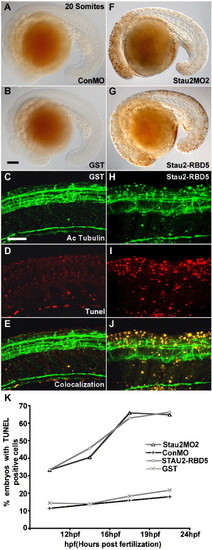
Cell death in the CNS of Stau2-depleted embryos. (A–D) TUNEL labeling showing dead cells in 20 somite stage embryos that were injected with control morpholino (A), GST (B, D), Stau2 MO2 (F), or Stau2 RBD5 (G, I). Immunostaining with an antibody towards acetylated tubulin to detect neurons (C, H) in embryos injected with GST (C–E) or Stau2 RBD5 peptide (H–J), and co-localization of acetylated tubulin with TUNEL positive cells (E, J). Lateral views, scale bar in panel B for panels A, B, F, G, 100 μm; panels C for panels C–E and H–J, 200 μm. (K) % embryos with TUNEL labeling at 12 hpf, 16 hpf, 19 hpf, and 24 hpf upon injection of control morpholino, stau2 morpholinos, GST peptide, or Stau2 RBD5 peptide. |
|
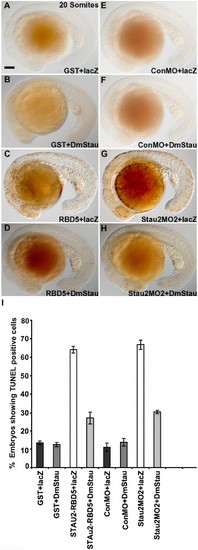
Drosophila melanogaster staufen rescues CNS cell death in Stau2-disrupted embryos. TUNEL labeling in embryos co-injected with GST (A, B), Stau2 RBD5 (C, D), control morpholino (E, F), or stau2 morpholino2 (G, H) and either lacZ RNA (A, C, E, G) or Drosophila melanogaster staufen RNA (B, D, F, H). Lateral views of 20 somite stage embryos. (I) % embryos with TUNEL-labeled cells. |
Reprinted from Developmental Biology, 292(2), Ramasamy, S., Wang, H., Quach, H.N., and Sampath, K., Zebrafish Staufen1 and Staufen2 are required for the survival and migration of primordial germ cells, 393-406, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.