- Title
-
Zebrafish penner/lethal giant larvae 2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis
- Authors
- Sonawane, M., Carpio, Y., Geisler, R., Schwarz, H., Maischein, H.M., and Nuesslein-Volhard, C.
- Source
- Full text @ Development
|
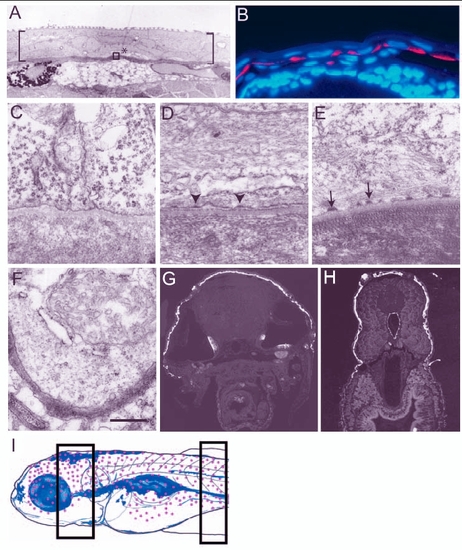
Establishment and maturation of basal domain of the basal epidermal cells during larval development. (A,C-F) Electron micrographs of epidermis. (B,G,H) Histological sections of 3- to 5-day-old larvae stained for keratin using Ks pan1-8 antibody (red in B and white in G,H; blue in B is the nuclear stain DAPI). (A) Larval epidermis, marked by brackets, is bilayered. Asterisk indicates the basal epidermal cell and the area equivalent to that included in the box was analysed from 3- to 5.5-day-old larvae to assess the formation of hemidesmosomes in C-E. (B) Keratin is localised basally in the basal epidermis of 3-day-old larvae. (C-E) Hemidesmosomes are not present in 3-day-old larvae (C); however, premature hemidesmosomes (arrowheads in D) and mature hemidesmosomes (arrows in E) are present in 4.5- and 5.5-day-old larva, respectively. (E,F) Although hemidesmosomes are present in the dorsal epidermis (E), they are absent in the ventral epidermis covering the lower jaw of 5.5-day-old larvae (F). Keratin is also absent in the ventral or ventrolateral epidermal cells of 5-day-old larvae (G,H). Schematic drawing (I) of 5.5-day-old larvae (based on whole-mount keratin staining, immunohistology and electron microscopy data), indicating the epidermal domain (red dots) marked by keratin expression and presence of hemidesmosomes. Sections analysed by electron microscopy were made from the body region marked with rectangles in I. Scale bar: 8.9 µm in A; 40 µm in B; 543 nm in C-F. |
|
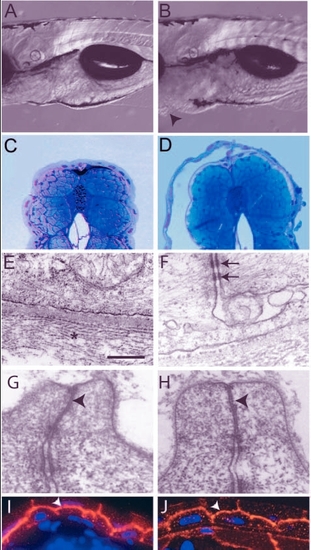
Analysis of the pen mutant phenotype. (A,B) DIC images of 5-day-old wild type (A) and pen mutant larvae (B). The pen mutant larvae exhibit small ventral epidermal growths (arrowheads in B). (C,D) Giemsa stained thin sections of 5-day-old wild-type and mutant larvae. Detachment of epidermis is observed in mutant larvae (D). (E-H) Electron microscopic analysis of the epidermis. Hemidesmosomes are absent and collagen lamella is non-coherent (star) but the basal lamina in between appears normal in the epidermis of pen mutant larvae (E; also compare with Fig. 1E). Desmosomes (arrows in F), however, are present in the mutant epidermis. Tight junctions in the periderm of wild-type and pen mutant (arrowheads in G and H, respectively) show no ultra-structural differences. (I,J) Immunostaining with anti-ß-catenin antibody to mark adherens junctions. In wild type (I) as well as in mutant (J), ß catenin (arrowheads) is localised at the apical and lateral borders of basal epidermal cells. Staining intensity is lower in the pen mutant. Scale bar: 0.3 mm in A,B; 160 µm in C,D; 543 nm in E; 405 nm in F; 297 nm in G,H; 30 µm in I,J. |
|
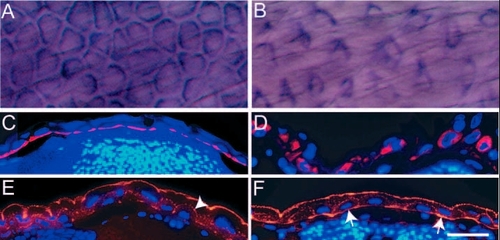
pen function is essential for the maintenance of the organisation of cytoskeletal elements in basal epidermal cells. (A-D) Immunohistological staining in 5-day-old wild-type (A,C) and pen mutant larvae (B,D) using anti keratin (Ks pan-8) antibody. In whole mounts, basal epidermal cells appear polygonal in wild type (A) and spindle shaped in mutants (B). Histological sections of 5-day-old wild-type and pen mutants stained for keratin counterstained by DAPI (C,D), show that in contrast to wild type (C), keratin (red) does not remain localised to the basal cortex in mutant larvae (D). (E,F) Immunohistological staining with anti-actin antibody followed by counterstaining with DAPI indicates punctate localisation of actin (red) at apical and lateral borders of basal epidermal cells in wild type (arrowhead in E). By contrast, actin is also localised basally in mutants (arrows in F). Scale bar: 40 µm in A,B; 60 µm in C-F. |
|
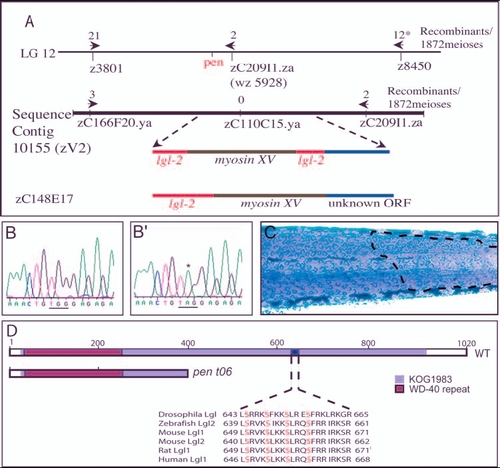
penner is the second zebrafish orthologue of D-lgl and is expressed in the basal epidermis. (A) pen maps in between SSLP markers z3801 and z8450 on linkage group 12. A BAC (zC209I1.za) that maps very close to pen also maps on sequence contig 10155 (zV2). pen shows tight linkage with a SNP marker present in zC110C15.ya (0 recombination). This region harbours putative myosin XV flanked by partial sequences homologous to lgl2. BAC zC148E17 maps to the same region and sequence analysis of this BAC revealed the presence of an uninterrupted lgl2 sequence. (B-B') The partial sequence of lgl2 from wild-type (B) and mutant (B') larvae. Asterisk in B' indicates the transition event leading to conversion of a codon for Trp (TGG in b) into a stop codon (TAG) in mutant. (C) pen mutant larvae injected with BAC zC242O17 containing wild type lgl2 and stained for keratin. BAC injections result in the partial rescue of pen mutant phenotype, as indicated by the presence of wild-type polygonal cells (marked by broken line) next to rounded up and loosely organised mutant cells. (D) Schematic of wild-type Lgl2, depicting WD-40 repeat and KOG 1983 domain. The sequence between amino acids 639 and 661 contains aPKC phosphorylation sites (serine residues in red) and is similar or identical to that in Drosophila Lgl, and Lgl 1 and Lgl2 of mouse, rat and human. There is a partial loss of KOG 1983 domain and a lack of aPKC phosphorylation sites in the pent06 allele. Asterisk indicates 776 meioses were analysed with SSLP marker z 8450. |
|
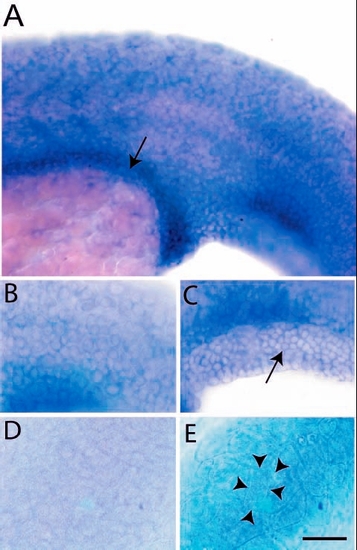
Expression analysis of lgl2 by in situ hybridisation. Epidermal cells and presumptive gut (arrow) of a 24-hour-old larva exhibit lgl2 expression (A). The basal cells of epidermis (B) including fin fold epidermis (C, arrow) express lgl2. Basal epidermis (D) and periderm (E) of control embryo hybridised with sense probe. The peridermal cells (cell borders marked by arrowheads in E) are bigger than the basal epidermal cells (B,C). Scale bar: 50 µm in A; 33 µm in B-E. |
|
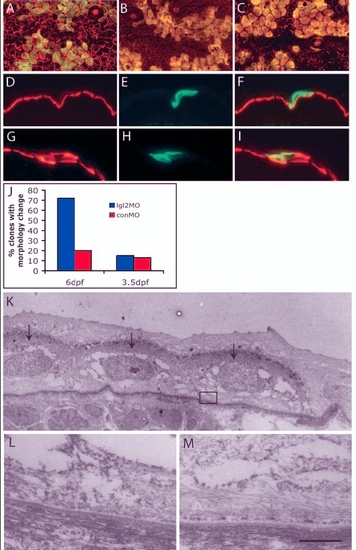
Clones derived from antisense lgl2 morpholino (lgl2MO)-injected embryos recapitulate the pen phenotype. (A-I) Confocal and immunohistological analysis of recipient embryos with epidermal clones labelled with GFP (green) and keratin (red). In overlays (A-C,F,I), clones appear yellow. At 6 dpf, clones derived from control morpholino (conMO)-injected embryos (A) comprise polygonal and flattened cells, similar to wild-type epidermal cells, while those derived from lgl2MO-injected embryos (B) showed changes in cellular morphology, becoming spindle or round shaped, similar to pen mutant. By contrast, on 3.5 dpf, clones containing lgl2MO (C) did not show any changes in cellular morphology. Immunohistological analysis of clones (6dpf) with conMO (D-F) reveals basal localisation of keratin, whereas clones containing lgl2MO exhibit mislocalisation of keratin (G-I). (J) Quantification of clones containing conMO or lgl2MO and exhibiting pen-like phenotype. Small proportions of clones carrying conMO comprise cells that deviate from usual morphology or show spindle shapes. There is a vast increase in the proportion of clones exhibiting pen-like phenotype at 6 dpf but not at 3.5 dpf (when, instead, they carry lgl2MO). (K-M) Electron microscopic analysis of hemidesmosome formation. Clone marked with GFP is detected with electron-dense DAB (arrows in K). Hemidesmosomes are absent in the clone (L represents boxed region in K) in contrast to the control recipient epidermis (M). Scale bar: 7 µm in K; 543 nm in L,M. |
|
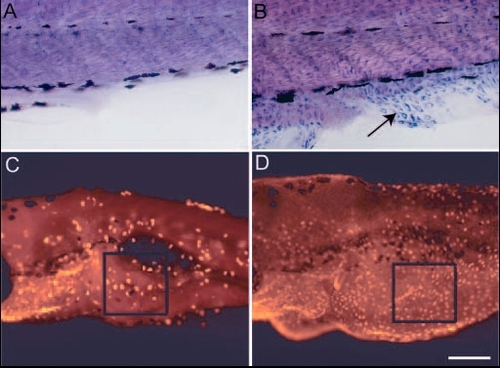
The loss of lgl2 function leads to acquisition of migratory behaviour and epidermal cell proliferation. (A,B) Immunohistological staining in wild-type (A) and pen mutant (B) using anti keratin (Ks pan-8) antibody. Epidermal cells exhibiting keratin staining are never present in fin fold of wild-type larvae (A). By contrast, mutant larvae show presence of keratin-containing cells in fin folds (arrow in B). These cells are spindle shaped and appear to migrate from the lateral epidermis into fin folds. (C,D) Assessment of cell proliferation using BrdU incorporation assay. The number of proliferating cells in ventral epidermis is higher in pen mutant (D) than in wild type (C). BrdU-labelled cells in the boxed region (in C,D) were counted from 10 different larvae to quantify the increase in pen larvae (see text for details). Scale bar: 40 µm in A,B; 0.2 mm in C,D. |
|
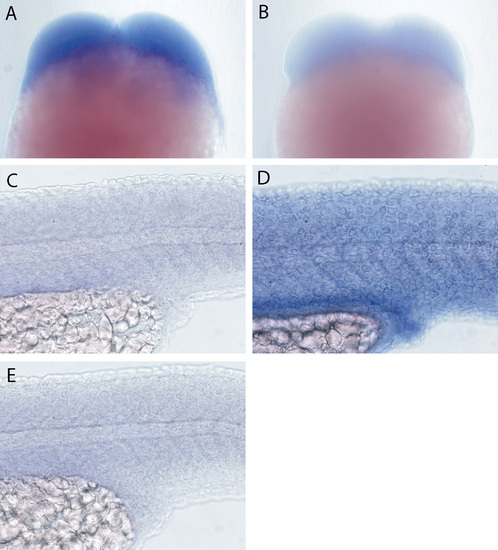
In situ expression analysis of lgl2 and lgl1. lgl2 is detected in embryos undergoing early cleavages (A). (B) Hybridisation with lgl2 sense control probe. lgl1 expression (C) was not detected in the epidermis and presumptive gut epithelium of 24-hour-old larvae. For comparison, lgl2 expression in the epidermis and presumptive gut epithelium is shown again in D. (E) Hybridisation with lgl1 sense control probe. |

Unillustrated author statements EXPRESSION / LABELING:
|