- Title
-
A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection
- Authors
- Xiao, T., Roeser, T., Staub, W., and Baier, H.
- Source
- Full text @ Development
|
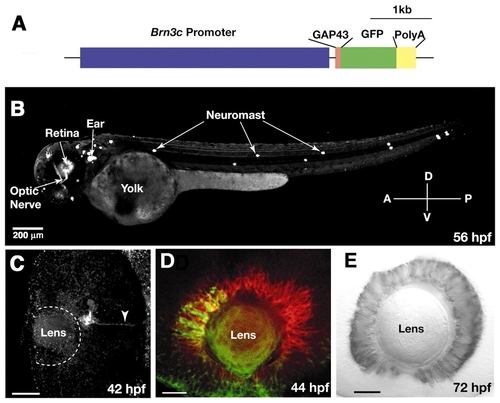
A large subset of RGCs express GFP in the stable Brn3c:mGFP transgenic line. (A) Schematic drawing of the DNA construct used to generate the Brn3c:mGFP transgenic line. (B) Lateral view of 56 hpf live Brn3c:mGFP transgenic embryo showing GFP expression by RGCs and mechanosensory hair cells (neuromasts) of the lateral line and inner ear. The optic nerve is visible. (C-E) Fixed Brn3c:mGFP transgenic embryos labeled by whole-mount immunohistochemistry. (C) Ventral view of a 42 hpf retina, labeled with anti-GFP. Anterior (nasal) is upwards. GFP expression starts in a small cluster of cells in the ventronasal retina, near the optic fissure. The arrowhead indicates the first GFP-positive axons exiting the eye through the optic stalk. (D) Lateral view of a 44 hpf retina, labeled with anti-GFP in green and zn5 in red. GFP has spread into central retina. The onset of Brn3c:mGFP transgene expression follows that of zn5 by 6 hours. (E) Lateral view of a 72 hpf retina, labeled with anti-GFP. GFP-positive RGCs are distributed uniformly throughout the ganglion cell layer. Scale bars: 200 µm in B; 20 µm in C-E.
|
|
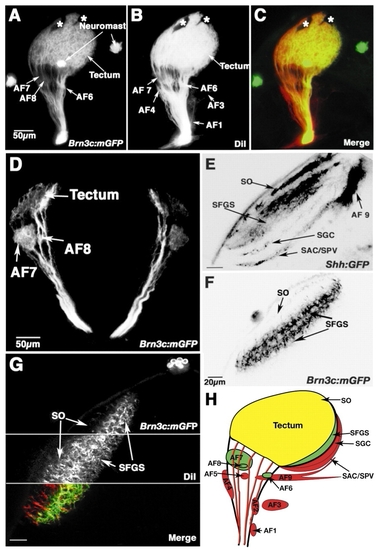
The Brn3c:mGFP transgenic line reveals the architecture of the retinotectal projection. (A-C) Lateral view of the retinofugal projection in a 6 dpf fixed larva, whose eye has been removed (projection of a confocal z-stack). Specimen was only lightly fixed to preserve GFP label. (A) Brn3c:mGFP transgene expression. Four arborization fields, AF-6, AF-7, AF-8 and tectum (AF-10), are visible, as well as neuromasts of the lateral line. (B) Retinofugal projection, labeled with DiI following intraocular injection. (C) Merged view of the two labels shown in A and B. (D) Transverse section of 7 dpf Brn3c:mGFP transgenic larva, showing AF-7, AF-8 and the tectal layers. (E-G) Optical sections of tecta in 6 dpf live larvae (dorsal view; anterior is upwards, midline is towards the right). (E) Shh:GFP labels all four retinorecipient layers in the tectum. (F) Brn3c:mGFP labels the SO and SFGS. (G) Double-labeling in vivo with DiI and Brn3c:mGFP, showing the absence of GFP in one of the three SFGS sublaminae. For technical reasons, live DiI staining is always incomplete. As a result, some GFP-only (green) fibers are seen in the SFGS. (H) Summary of the Brn3c:mGFP labeling pattern. RGCs project to ten AFs. The largest AF, the tectum, has four layers. Only the green areas receive significant Brn3c:mGFP input. Red areas are devoid of GFP label. Yellow area (SO) is innervated mainly by non-GFP fibers, but also receives weak Brn3:mGFP innervation. AF, arborization field; SO, stratum opticum; SFGS, stratum fibrosum et griseum superficiale; SGC, stratum griseum centrale; SAC, stratum album centrale; SPV, stratum periventriculare; asterisks, melanophores in the skin. Scale bars: 50 µm in A-D; 20 µm in E-G.
|
|
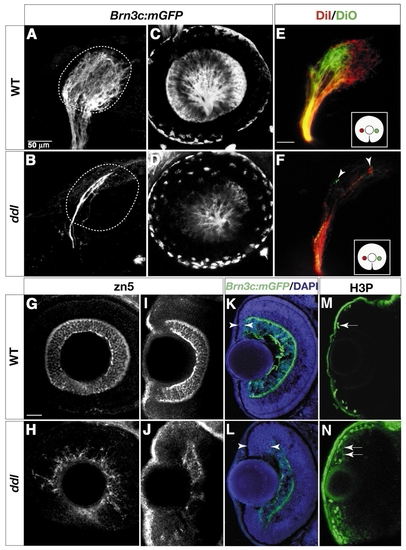
daredevil (ddl) mutants have fewer RGCs. Analysis of cell-type markers in wild type (A,C,E,G,I,K,M) and ddl (B,D,F,H,J,L,N). (A,B) Lateral views of 78 hpf tecta, labeled with whole-mount anti-GFP. Broken lines outline boundaries of the tectal neuropil. The wild-type tectum (A) is covered by axons. Few axons can be detected in the ddl tectum (B). (C,D) Confocal images of retinas in live 60 hpf embryos. The number of GFP-positive RGCs is greatly reduced in ddl (D). (E,F) Analysis of the retinotopic map in 72 hpf wild type (E) and ddl (F). DiI (red) and DiO (green) were pressure injected into nasal and temporal retina, respectively (see inserts for illustration of retinal injection sites). The gross topography of axon targeting in ddl mutants is not affected, with nasal axons still projecting to the posterior tectum and temporal axons to the anterior tectum (F). (G-J) Whole-mount Zn5 staining of 72 hpf retinas. (G,H) Lateral views. (I,J) Dorsal views. The number of zn5-positive RGCs is greatly reduced in ddl. (K,L) Sections of 78 hpf retinae, labeled with anti-GFP (green) and DAPI (blue). The ciliary margin (between arrowheads) in the ddl retina is wider than in wild type. (M,N) Dorsal views of 72 hpf whole-mount retinae, labeled with anti-phosphohistone H3 (H3P). The number of dividing cells (arrows) is greatly increased at the ciliary margin in ddl. Scale bars: 50 µm in A and E (for tectum panels); 20 µm in G (for retina panels).
|
|
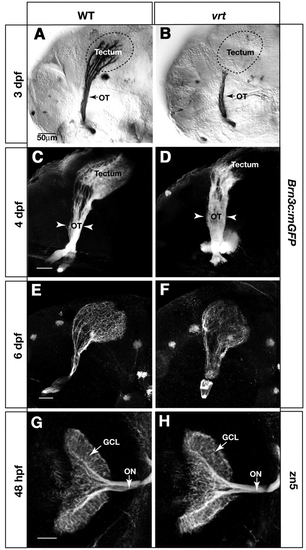
vertigo (vrt) mutants show severely delayed innervation of the tectum. Analysis of retinal axon projection in wild type (A,C,E,G) and vrt mutants (B,D,F,H). (A-F) Lateral views of the retinotectal projection in Brn3c:mGFP transgenic fish, labeled with anti-GFP. At 3 dpf, the wild-type tectum (A) is fully innervated, while the vrt tectum (B) is devoid of axons. Broken lines outline the tectum boundaries. In 4 dpf wild-type larvae (C), the density of axon arbors is increased compared with 3 dpf (A), and dorsal and ventral branches are clearly visible in the optic tract. In vrt (D), axons have invaded the anterior tectum, and the optic tract (OT) is abnormally wide (arrowheads). At 6 dpf, axons innervate the whole tectum in the vrt mutant (F) similar to wild type (E) The optic tract remains wider than normal. (G,H) Dorsal views of 48 hpf retinas and optic nerves, labeled with zn5. The number of RGCs and their axons is similar between wild type (G) and vrt (H). Scale bars: 50 µm.
|
|
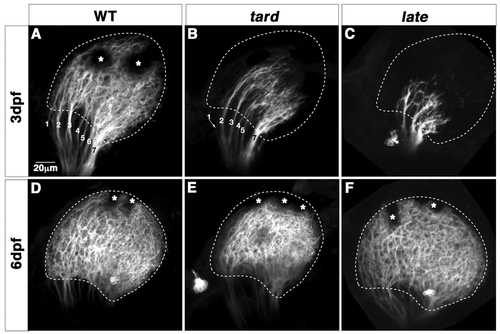
tarde demais (tard) and late bloomer (late) mutants show mildly delayed innervation of the tectum. (A-F) Confocal images of Brn3c:mGFP labeled retinotectal projections of 80 hpf tecta in live larvae (A-C). The wild-type tectum (A) is filled with retinal axons. The optic tract has branched into stereotyped fascicles, labeled with numbers. The tard tectum (B) and the late tectum (C) are less than halfway innervated. (D-F) Lateral views of 6 dpf tecta. The tard tectum and the late tectum are now covered with axons. However, the tard tectum is small and its boundary remains abnormal (E), compared with wild type (D) and in contrast to late (F). Asterisks indicate melanophores on the skin. Broken lines outline the tectal neuropil. Scale bars: 20 µm.
|
|
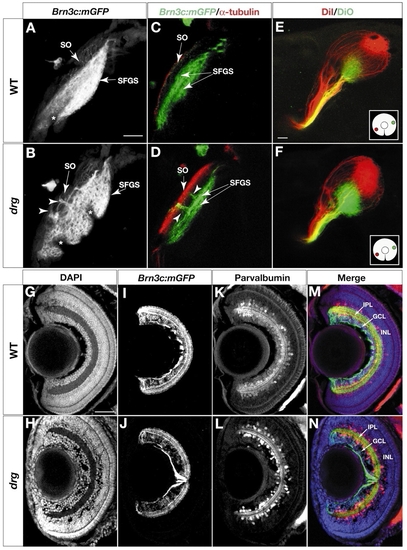
Laminar specificity is perturbed in dragnet (drg) mutants. (A-D) Analysis of retinorecipient layers in 6 dpf wild type (A,C) and drg (B,D). (A,B) Z projections of confocal image stacks, labeled with Brn3c:mGFP. Larvae are mounted at a slightly oblique angle to better visualize the gap between SO and SFGS. (C,D) Optical sections of Brn3c:mGFP tecta, stained with whole-mount anti-acetylated tubulin (red) and anti-GFP (green). Arrowheads indicate ectoptic axon fascicles traveling between SO and SFGS in drg mutants. (E,F) Analysis of the retinotopic map in 6 dpf wild type (E) and drg (F). DiI (red) and DiO (green) were pressure injected into ventronasal and dorsotemporal retina, respectively (see inserts for illustration of injection sites). Axon targeting in drg (F) is comparable with wild type (E), suggesting that positional information along the retinotopic axes is intact. (G-N) Analysis of the inner plexiform layer (IPL) in sections of 6 dpf retina (see M,N for labels of retinal layers). (G,H) DAPI labeling. (I,J) Anti-GFP labeling to visualize the four sublaminae to which Brn3c:mGFP RGC dendrites project. (K,L) Anti-parvalbumin labeling, to highlight a subpopulation of amacrine cells that project to three sublaminae in the IPL. (M,N) Triple-labeling (merged image) showing DAPI (blue), anti-GFP (green) and anti-parvalbumin (red). Formation of IPL sublaminae is not affected in drg. An apparently unrelated phenotype of drg can be seen using the DAPI stain: an abnormal aggregation of cells in front of the drg lens (H). Asterisks indicate melanophores in the skin. Scale bars: 20 µm in A,G.
|
|
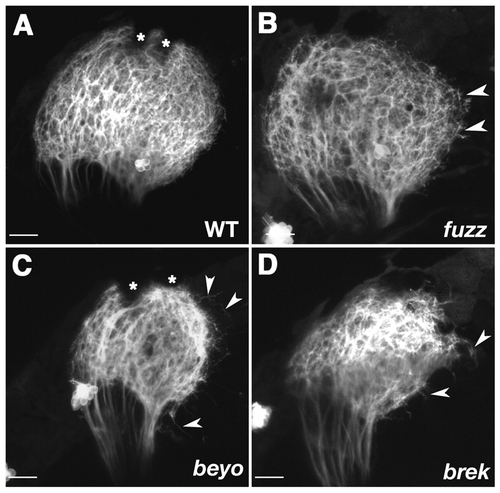
Tectal neuropil boundaries dissolve in fuzz wuzzy (fuzz), beyond borders (beyo) and breaking up (brek) mutants. (A-D) Z-projections of confocal image stacks showing lateral views of tecta in 6 dpf live Brn3c:mGFP larvae. The boundary of the tectum is smooth and well defined in wild type (A). In fuzz (B), axon arbors are less dense in the tectal neuropil and often overshoot the tectal boundary (arrowheads). The overshooting phenotype is more severe in beyo (C) and brek (D). Asterisks indicate melanophores. Scale bars: 20 µm.
|
|
Fasciculation is disorganized in blue kite (bluk) and coming apart (coma), blue kite (bluk), clewless (clew) and blind date (blin) mutants. (A-F) Surface-rendered 3D reconstructions of confocal images taken from 6 dpf live Brn3c:mGFP larvae. Dorsal views (A,C,E,G,I) and mirror-image ventral views (B,D,F,H,J) of the same tecta are shown. On the dorsal surface, axon fascicles are visible within the tectal neuropil of wild type (A) (arrowheads). The ventral view (B) demonstrates the characteristic dense grid of arbors. (C,D) In coma, fascicles both in the optic tract (hatched rectangle in D) and in the neuropil are disorganized. (E-J) In bluk, clew and blin, fascicles within the neuropil are greatly reduced (E-I) and axon termations appear diffuse. Asterisks indicate melanophores in the skin. Scale bars: 20 µm.
|
|
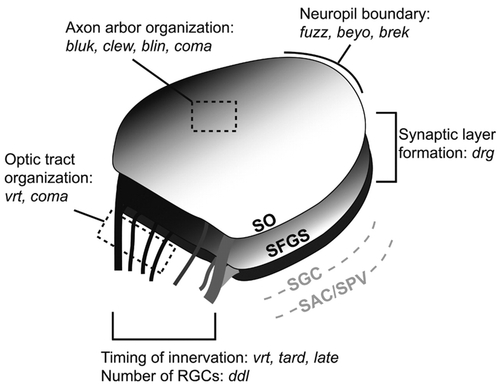
Summary of retinotectal mutants. The 13 loci are predicted to affect different aspects of tectal development and architecture. The ddl protein is required for differentiation of most RGCs. The vrt, tard and late gene products ensure timely innervation of the tectum. Products of vrt and coma organize the fascicles in the optic tract, while bluk, clew, blin and coma regulate fiber-fiber interaction in the tectum. Proteins encoded by fuzz, beyo and brek confine axons to the neuropil, while drg is required for targeting axons to the SFGS. (The deeper layers of the tectum, SGC and SAC/SPV, are not labeled by Brn3c:mGFP.)
|
|
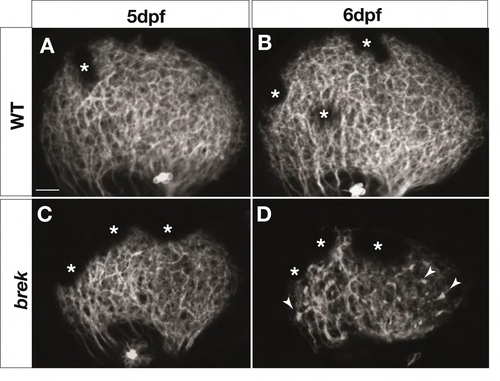
Retinal axons degenerate in breaking up (brek) mutants. Z-projections of confocal image stacks showing lateral views of the tectum in 5-6 dpf live Brn3c:mGFP larvae. At 5 dpf, retinal axons cover the tectum normally in brek mutants (C), similar to wild type (A). At 6 dpf, retinal axons become punctate in the brek tectum and coverage is more sparse (D) than in wild type (B). Arrowheads indicate puncta. Asterisks indicate melanophores in the skin. Scale bars: 20 mm. |
|
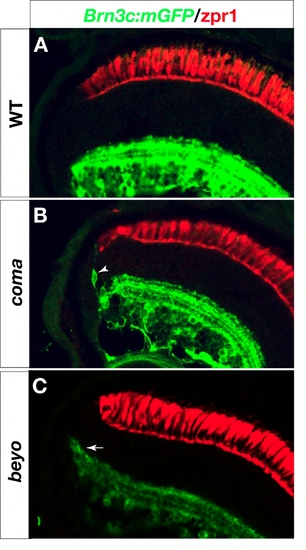
Retina defects in coming apart (coma) and beyond borders (beyo) mutants. Six dpf retina sections labeled with anti-GFP in green and zpr1 in red (A-C). (B) In coma, dendrite-bearing, GFP-positive neurons (arrowhead) are found at the margins of the inner nuclear layer. (C) In beyo, the edge of the inner plexiform layer ?smiles?, i. e. it bends toward the outer retina (arrow). |