- Title
-
pitx3 defines an equivalence domain for lens and anterior pituitary placode
- Authors
- Dutta, S., Dietrich, J.E., Aspock, G., Burdine, R.D., Schier, A., Westerfield, M., and Varga, Z.M.
- Source
- Full text @ Development
|
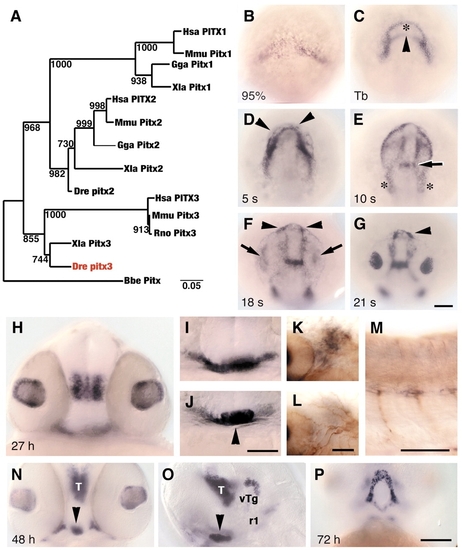
Zebrafish lens and pituitary cells express Pitx3. (A) Nearest-neighbor joining tree of full-length Pitx amino acid sequences. Zebrafish Pitx3 (red) groups with Xenopus and mammalian Pitx3 proteins. Bootstrap analysis: random number seed, 111; numbers on branches indicate the number of times each node was obtained in 1000 runs. Danio rerio (Dre, AF181681), Homo sapiens (Hsa, P78337, AF238048, AF041339), Mus musculus (Mmu, NM_011097, NM_011098, NM_008852), Rattus norvegicus (Rno, NM_019334, NM_019247), Gallus gallus (Gga, AF069397, AF076640), Xenopus laevis (Xla, AF217647, AF077767, AF297713) and Branchiostoma belcheri (Bbe, AF195616). (B-J) In wild-type embryos, pitx3 is expressed (black) at (B) 95% epiboly; (C) bud stage, in two domains that outline underlying polster (asterisk) and anterior neural plate border (arrowhead); (D) five-somite stage, in cells (arrowheads) around the anterior neural keel; (E) 10-somite stage, presumptive first branchial arch precursors (asterisks) and presumptive forebrain-midbrain border (arrow); (F,G) 18- and 21-somite stages, in prospective lens (arrows) and pituitary placodes (arrowheads); (H) 27 h, in lens and ventral posterior diencephalon; (I) 27 h, in ventral head mesenchyme; (J) 27 h, pituitary (arrowhead). (K-M) 27 h. Double labeling with mRNA probe for pitx3 (black) and anti-acetylated tubulin (brown) indicates that branchial arch mesenchyme (K), which is posterior to eye and ventral to trigeminal placode (L), and trunk muscle pioneer cells (M) express pitx3. (N-P) At 48-72 h, cells in pituitary (arrowheads), thalamus (T), ventral tegmentum (vTg), first rhombomere (r1) and Meckel′s cartilage (P) express pitx3. (B-G) Dorsal view of prospective head region, ventral towards the top; (H-J,N) frontal view, dorsal upwards; (K,L) dorsal view, anterior towards left, different focal planes of same region; (M,O) side view, anterior towards left and dorsal towards top; (P) ventral view, anterior towards top. Scale bars: 100 µm in B-G,H-J,N-P; 50 µm in K-M. EXPRESSION / LABELING:
|
|
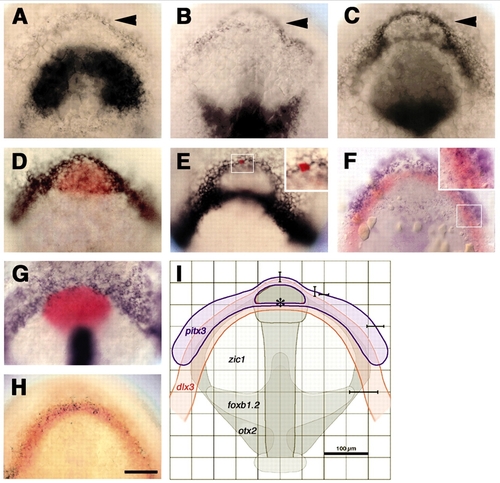
pitx3 and dlx3b form overlapping expression domains at the anterior neural plate border. (A-D) Embryos labeled with probes for pitx3 (arrowheads) and (A) zic1, (B) foxb1.2; (C) pax6a or (D) hgg1 (red). (E) Anterior neural plate border cells express dlx3b. (E, inset) Single cell injected with fluorescein-dextran, labeled for lineage tracer (red). (F-H) A subset of cells co-expresses: (F) pitx3 (blue) and dlx3b (red); (G) bmp2b (blue, non-neural ectoderm), shh (blue, neural plate midline) and hgg1 (red, polster); (H) dlx3b (red) and eya1 (black). (I) Sketch of anterior neural plate gene expression domains (pitx3, blue, n=12; dlx3b, red, n=15; zic1, foxb1.2, otx2, grey) (Varga et al., 1999) to predict location of pitx3-expressing cells in live unlabeled embryos. Averaged gene expression domains; bars indicate standard deviations from the mean, relative to neural plate midline and center of polster (asterisk). All panels at bud stage, except C, which is at the one-somite stage. Dorsal views of prospective head region, ventral towards the top. Scale bars: 100 µm.
EXPRESSION / LABELING:
|
|
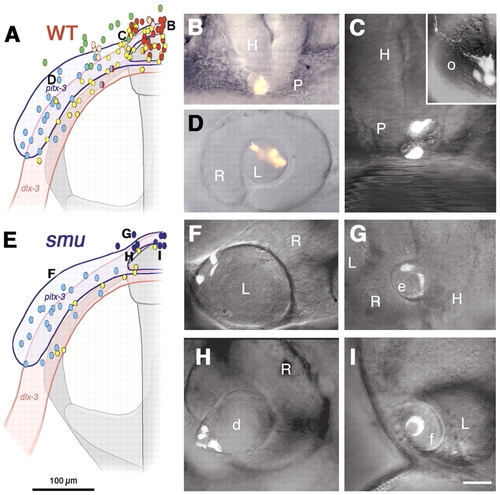
Pituitary precursors form lens in smoothened mutant embryos. (A-D) Wild-type embryos, (E-I) smu mutant embryos. Colored dots and letters in A,E represent locations of injected precursor cells at bud stage and their fates at prim-5 stage (B-D,F-I). (A) Fate map of wild-type embryo (n=105) shows pituitary precursor cells (red) at anterior midline (B, n=22), lens precursor cells (blue) at lateral neural plate border (D, n=21) and olfactory placode precursors (yellow) closer to neural plate (C, inset, n=37). (C) Split fates from a single precursor cell (yellow/red dots in A) contribute to pituitary and olfactory placode. Green dots, epidermal precursors; pink dots, head mesenchymal precursors. (E) Fate map of smoothened mutant embryo (n=33). Precursor cells give rise to ectopic median (dark blue) or normal retinal lenses (light blue); yellow, olfactory placode precursors (n=9). (F) Precursor cells from lateral pitx3 domain (light blue) give rise to lens epithelial and primary lens fiber cells. (G-I) Median placodal precursors give rise to ectopic (G), distorted (H) or fused (I) lens, and not to pituitary as expected from median positions in wild-type embryos (A, red). Ventral ectoderm and head mesenchyme cells not shown for clarity. d, distorted lens; e, ectopic lens; f, medially fused lens; H, hypothalamus; L, retina associated lens; o, olfactory placode; P, pituitary; R, retina. (A,B) Dorsal view, prospective head, anterior towards the top (compare with Fig. 2I). (B,C,G) Frontal view, dorsal towards the top. (D,F,H,I) Side view, anterior towards the left. Scale bar: 100 μm in A,E; 50 μm in B,D,E; 25 μm in C,F-I. |
|
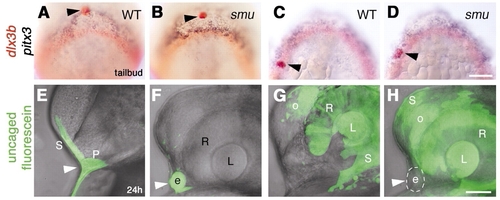
Ectopic lens formation in smoothened mutant embryos does not result from aberrant movement of lens precursors. (A-D) Bud stage embryos fixed immediately after photoactivation, then hybridized with probes for pitx3 (blue) and dlx3b (red). Photoactivated median anterior cells (A,B; n=14) and lateral posterior cells (C,D; n=11) in wild-type (A,C) and smoothened mutant (B,D) embryos labeled with anti-fluorescein (red, black arrowheads). (E-H) Embryos at prim-5 stage, after uncaging at bud stage as indicated in A-D. (E) Wild-type; skin and anterior pituitary placode are labeled (n=10; white arrowhead). (F) smoothened mutant; ectopic lens (white arrowhead) and skin (F; n=7) are labeled (photoactivation as indicated in A,B). (G,H) Wild-type (G; n=19) and smoothened mutant (H; n=7), skin, lens, retina and olfactory placode labeled (photoactivation approximately as indicated in C,D). (H) Ectopic median lens (white arrowhead, circle) is not labeled in smoothened mutant embryos (n=7). More cells were labeled in G,H than indicated in control embryos (C,D). (E-H) Superimposed bright-field images and fluorescent confocal stacks. e, ectopic lens; L, retina-associated lens; o, olfactory placode; P, pituitary; R, retina; S, skin;. (A-D) Dorsal views of prospective head region, anterior towards top; (E-H) side views, anterior towards the left, dorsal towards the top. Scale bars: 100 μm in A-D; 50 μm in E-H. |
|
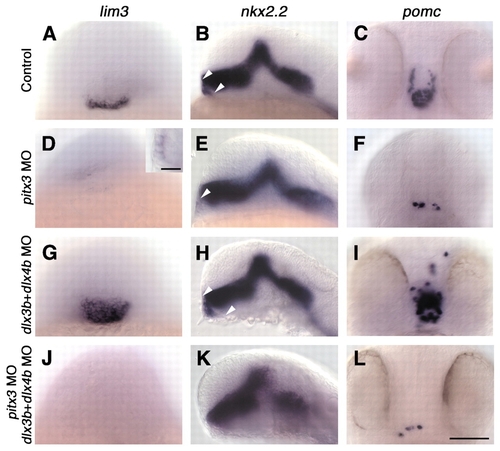
pitx3 is required for pituitary placode formation, whereas dlx3b and dlx4b control pituitary placode size. Wild-type (A-C) and morpholino (MO)-injected (D-L) embryos labeled with probes for lim3 (A,D,G,J), nkx2.2 (B,E,H,K) and pomc (C,F,I,L). (D-F) Injection of pitx3 morpholinos (n=94) leads to loss of pituitary placode (20/94 embryos) or severe reduction in pituitary cell number (D, inset; n=69/94). (G-I) Injection of dlx3b and dlx4b morpholinos (n=103) leads to enlarged pituitary placodes and increased pituitary cell numbers (n=78/103), arrowheads in B,E,H. (J-L) Injection of pitx3, dlx3b and dlx4b morpholinos (n=83) leads to loss of pituitary placode (42/83 embryos) or severe reduction in pituitary cell number (n=25/83). (A,C,D,F,G,I,J,L) Frontal views, dorsal towards the top; (B,E,H,K) side views, anterior towards the left. Scale bars: 100 µm in A-L; 25 µm in D, inset.
EXPRESSION / LABELING:
|
|
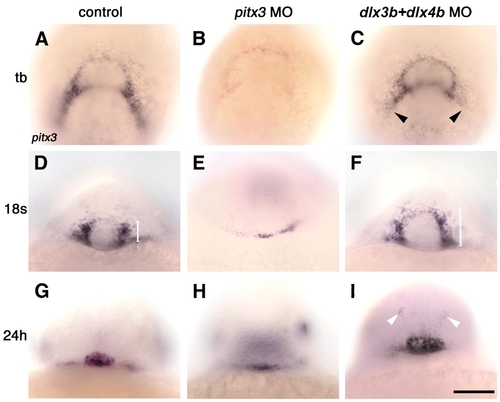
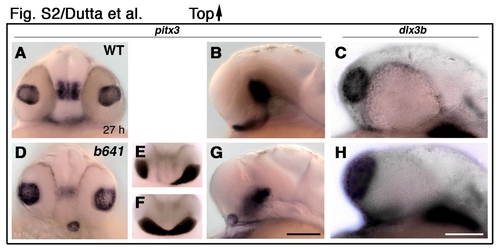
Pitx3 is required for pitx3 expression at bud stage, and Dlx3b and Dlx4b restrict pituitary pre-placode size during mid-somitogenesis. (A-I) Control and morpholino injected embryos, labeled with pitx3 probe at bud stage (tb, A-C), 18-somite stage (D-F) and prim-5 stage (24 h; G-I). (B,E,H) pitx3 morpholino injection leads to reduced pitx3 expression in placodal field (B; n=23/30), in pituitary pre-placode (E; n=33/46) and in pituitary placode (H; n=43/68). (C,F,I) Injection of dlx3b and dlx4b morpholinos leads to reduction of pitx3 expression in prospective lens precursors (arrowheads in C; n=29/38), and an expansion of the pituitary pre-placode (F, white bar; n=23/30) and pituitary placode size (I; n=32/40; arrowheads: ectopic pitx3 expression). (A-C) Dorsal view, ventral towards the top. (D-I) Frontal view, dorsal towards the top. Scale bar: 100 µm.
|
|
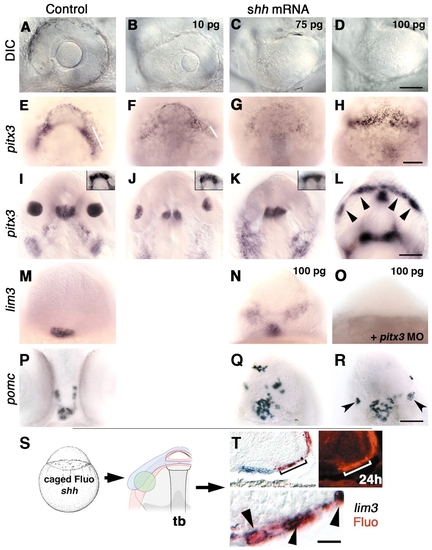
Hedgehog can block lens formation and induce gene expression characteristic of pituitary cell types. (A-L) Increasing amounts (10, 75 and 100 pg) of injected shh mRNA affect lens size at prim-5 stage (A-D) and pitx3 gene expression at bud (E-H) and prim-5 (I-L) stages in a dose-dependent manner. (B,J) Reduced lens size (n=54/60). (C,K; n=52/60; D,L; n=93/99) Absence of lens tissue. (I-K) Pituitary placode size at prim-5 stage is unaffected by hedgehog mRNA (I-K insets; n=93/99). (E-G) At bud stage, progressive loss of pitx3 expression in presumptive lens precursors (bars; F, n=10/14; G, n=11/12) correlates with loss of lens tissue at prim-5 stage (B,C,J,K). (L) Ectopic pitx3 expression following injections of 100 pg shh mRNA per embryo at bud stage in anterior ventral ectoderm (H, 8/8) and at prim-5 stage (L, arrowheads; n=15/23). (M,N;P-R) shh mRNA induces ectopic lim3 (N; n=30/36) and pomc (Q,R; n=26/32). (Q) Ectopic pomc-expressing cells are scattered around forebrain (n=19/26) or (R, n=7/26) form small lens like structures (arrowheads). (O) Co-injection of pitx3 morpholino (8 ng/embryo) and shh mRNA (100 pg) leads to loss of lim3 expression in pituitary and ectopic lim3-expressing regions (n=28/30). (S) One-cell stage embryos injected with shh mRNA and caged fluorescein (n=27). Photoactivated spot of cells (green) at bud stage in the lens-forming region. (T) In shh mRNA-injected embryos, ectopic lim3 (n=9)-expressing cells (blue) were also labeled with uncaged fluorescein (red, arrowheads). Upper left and lower (magnification of upper left) panels, Nomarski images; upper right panel, fluorescence micrograph of same region showing fast red fluorescence in ectopic lim3-expressing cell. (A-D) Side views, anterior towards the left, dorsal towards the top. (I-L, insets) Dorsal views, anterior towards the top. (E-H) View of prospective head region (animal pole), ventral towards the top. (M-R) Frontal views, dorsal towards the top. (T) prim-5 stage embryo, transverse cryosection (16 µm thickness) through ventral forebrain. Scale bars: 50 µm in A-D; 100 µm in E-L, insets, M-R; 12.5 µm in T, lower panel.
|
|
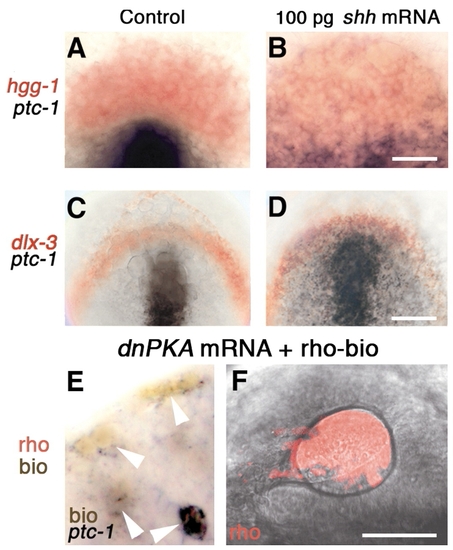
At bud stage, lens and pituitary precursor cells do not upregulate ptc1 in response to shh mRNA. (A-D) Bud stage embryos labeled with probes for (A,B) hgg1 (red) and ptc1 (black), (C,D) dlx3b (red) and ptc1 (black). (E) At the 18-somite stage, dnPKA mRNA and rhodamine-biotin injected, transplanted cells are ptc1 positive (arrowheads) in ectoderm of eye vesicle in unlabeled host (n=11). (F) At prim-5 stage, transplanted cells (rho, red) contribute to lens (n=27). (A-D) Dorsal view of prospective head region, ventral towards the top. (E) Dorsal view, anterior towards the top, midline towards the right. (F) Side view, anterior towards the left, dorsal towards the top; confocal fluorescence micrograph reconstructed from 90 optical sections (optical slice 1.6 μm; 0.25 μm overlap between consecutive sections). Scale bars: 100 μm in C,D; 50 μm in A,B,F; 12.5 μm in E. EXPRESSION / LABELING:
|
|
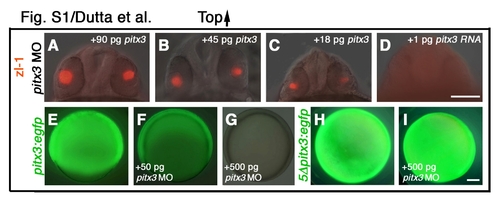
pitx3 mRNA translation is blocked by pitx3 morpholinos. (A-D) pitx3 mRNA rescues pitx3 morpholino-injected embryos (8 ng morpholino/embryo). (E-G) Embryos co-injected with increasing doses of pitx3 morpholino and pitx3:eGFP mRNA (mRNA from 5′UTR of pitx3 cDNA linked to eGFP; 300 pg/embryo). Morpholinos suppress eGFP fluorescence in a dose-dependent manner (F,G). By contrast, eGFP fluorescence is not blocked by pitx3 morpholino in embryos injected with a modified mRNA construct 5Dpitx3:egfp that contains five mismatches in the morpholino-binding site of the pitx3 5′ UTR (I). (A-D) Frontal view, dorsal towards the top; (E-I) Side views, animal pole towards top. Scale bars: 200 μm in A-D; 100 μm in E-I. |
|
Hedgehog signaling is required for pituitary cell specification and regulates olfactory placode size. Reduced Hedgehog signal transduction increases lens and olfactory placode domains, while reducing pituitary placode specification. (A,B) pitx3 expression in wild-type embryos. (C) dlx3b expression in wild-type embryos. Eighty-nine percent of smoothened mutants (n=83) have ectopic and retina-associated lenses (D,G), 7% have distorted lens (E) and 4% have fused lens (F). In smoothened mutants, we observe normal pitx3 expression in anterior neural plate border ectoderm at bud stage (not shown), and in lens tissue at prim-5 stage (D-G). By contrast, head mesenchyme and branchial arches fail to express pitx3 and expression in ventral forebrain is reduced (D,G). (C,H) In 97 smoothened mutant embryos, 5% had larger olfactory placodes (H) than wild-type embryos (C). Although the olfactory placodes never fuse across the ventral midline, this phenotype correlates with the extended and fused lens placodes in smoothened mutants. (A,D-F) Frontal view, dorsal is upwards; (B,C,G,H) side views, anterior towards the left, dorsal towards the top. Scale bars: 100 μm EXPRESSION / LABELING:
|
|
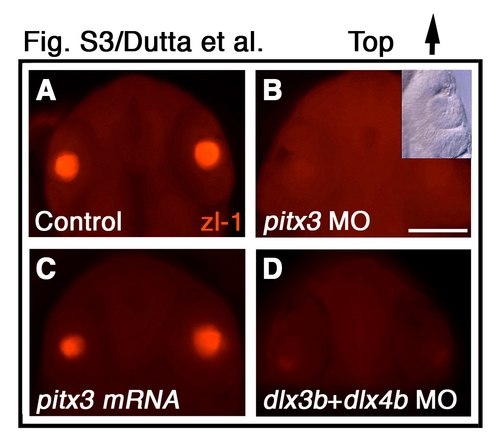
pitx3 and dlx3b are required for primary lens fiber cell differentiation. (A-D) zl-1 immunofluorescence labeling of primary lens fiber cells (red) in (A) control, (B) pitx3 morpholino-injected, (C) pitx3 mRNA-injected, and (D) dlx3b and dlx4b morpholino-injected embryos. Inset in B shows that lens form in presence of pitx3 morpholino. (A-D) Frontal view, dorsal towards the top. MO, morpholino. Scale bar: 200 μm. |