- Title
-
Intestinal growth and differentiation in zebrafish
- Authors
- Wallace, K.N., Akhter, S., Smith, E.M., Lorent, K., and Pack, M.
- Source
- Full text @ Mech. Dev.
|
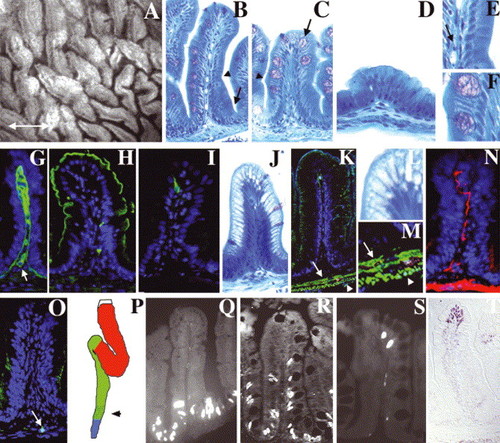
Adult zebrafish intestine: (A) Whole-mount, interior view of the adult zebrafish anterior intestine reveals irregularly shaped epithelial folds. The long axis of most folds is oriented circumferentially; others are oriented randomly (double arrow defines anterior–posterior axis). (B–D) Histological cross-section through the folds of the anterior (B), mid (C) and posterior (D) intestine. Note progressive diminution of the height of mid and posterior folds. A connective tissue core containing blood vessels and nerves underlies the epithelium of each fold. Note absence of crypts at base of folds (arrow in B). (E,F) Enlargement of region adjacent to arrowheads in (B,C) showing columnar-shaped enterocytes and goblet cells. Arrow in (E) points to an erythrocyte in a blood vessel in the connective tissue of the intestinal fold (G) Laminin immunohistochemistry identifies the basement membrane separating the epithelium from the underlying connective tissue (lamina propria). Laminin also encircles blood vessels within the lamina propria (arrow). Four principal cell types are identified within the epithelium. (H) Enterocytes are identified by apical sodium phosphate transporter (NaPi). (I) Enteroendocrine cells are identified by pancreatic polypeptide. (C,F) Goblet cells are identified histologically by their mucinous theca (arrow in C). (J,L) Antigen presenting cells within segment II of the mid-intestine are identified by their distinctive supra-nuclear vacuole. (K,M) Desmin (green) identifies inner circular (arrow) and outer longitudinal (arrowhead) smooth muscle layers beneath the lamina propria. (N) Acetylated tubulin identifies axonal fibers within the muscularis and the lamina propria. (O) Hu immunohistochemistry identifies a solitary enteric neuron cell body within the muscularis (arrow). (P) Schematic representing the anterior (red), mid (green) and posterior (blue) intestinal segments. Stippled region of the mid segment represents location of specialized enterocytes. (Q–S) Intestinal epithelial cell renewal revealed by BrdU immunohistochemistry. S-phase cells are present at the base of the intestinal folds 1 h after BrdU injection (Q). 4-day post-injection, S-phase cells are identified at the base and in the mid fold region (R). 7-day post-injection, S-phase cells are only identified at the tip of the folds (S). (T) TUNEL assay identifies apoptotic cells at the tips of intestinal folds. |
|
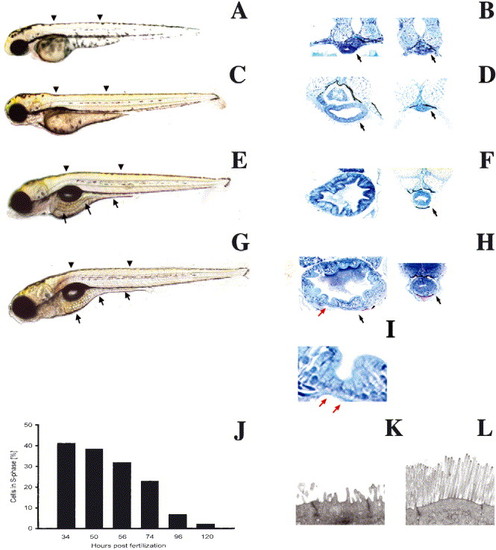
Biphasic intestinal growth in zebrafish embryos and larvae: (A–D) Lateral views of 50, 74, 96 and 120 hpf zebrafish. The intestine is prominent at 96 and 120 hpf (arrows C, D) but difficult to identify at 50 (A) and 72 hpf (B). (E–H) Corresponding histological cross-sections through the anterior (left) and posterior (right) intestine of zebrafish depicted in (A–D). Note appearance of folds and columnar-shaped epithelial cells between 72 and 96 hpf. Folds are cut in cross-section (red arrow) and tangential to the long fold axis (black arrow). (I) High power view of intestinal folds in H shows cuboidal cells at base of fold (red arrow) and adjacent columnar cells. (J) Epithelial cell proliferation is biphasic; nadir is reached at 120 hpf and cell proliferation does not increase within the first 24 hpf of feeding (not shown). Arrowheads identify levels of histological sections. Arrows in histological sections point to the intestine unless otherwise noted. (K,L) Transmission electron micrographs showing the apical region of a 74 (K) and 120 hpf (L) intestinal epithelial cell. |
|
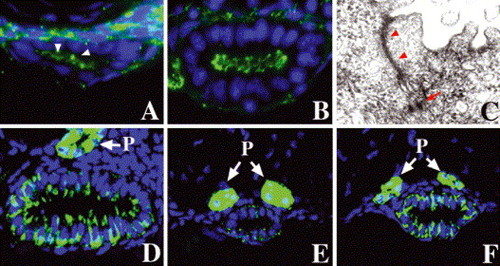
Step-wise polarization of developing intestinal epithelia: (A) cross-section through the anterior intestine of a 50 hpf embryo processed for ZO-1 immunohistochemistry. Apical ZO-1 (arrowheads) is first evident at this stage. (B) Apical ZO-1 staining in the anterior intestine is pronounced at 74 hpf. (C) Transmission electron micrograph (TEM) shows well-defined tight junction (arrowheads) and desmosomes (arrow) in an intestial epithelial cell of a 74 hpf larva. (D–F) Basolateral Na/K–ATPase is present in the 74 hpf anterior intestinal epithelium, but there is minimal staining in the posterior intestine at this stage (E). Pronounced Na/K–ATPase is present in the posterior intestine at 5 dpf (F). (P) Pronephric ducts. |
|
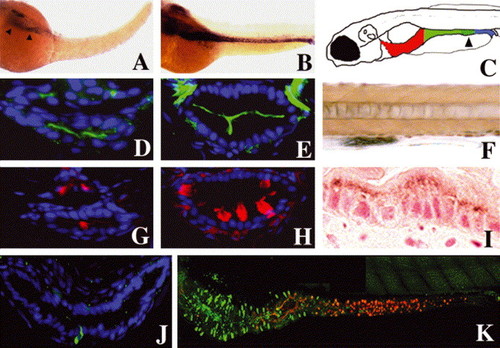
Smooth muscle progenitors and epithelial differentiation: (A,B) Whole-mount RNA in situ showing intestinal smooth muscle myosin heavy chain expression at 50 (A, arrowheads) and 58 hpf (B). (C) Cartoon depicting anterior (red), mid (green) and posterior (blue) segments of the 5 dpf larval intestine. Distal region of the mid intestine that contains specialized enterocytes is stippled (arrowhead). (D,E,G,H) Cross-sections through the larval mid intestine. (D,E) Enterocytes contain the NaPi transporter within their apical cell membrane. NaPi+ enterocytes are first identified at 74 hpf (D); NaPi levels are pronounced at 120 hpf (E). (G) Goblet cell mucin, revealed here by rhodamine-dextran labeled WGA, is first evident at 78 hpf. (H) Mucin is much more abundant at 120 hpf and is easily recognized in histological specimens at this stage (not shown). (J) Cross-section through the anterior intestine of a 96 hpf larva. Pancreatic polypeptide+ enteroendocrine cells are first identified at this stage. Note slender apical cytoplasm characteristic of these cells. (K) Lateral view of the 120 hpf intestine showing regionalized distribution of differentiated epithelial cells. Pancreatic polypeptide containing enteroendocrine cells (green) are restricted to the anterior intestine (intestinal bulb) whereas goblet cells identified with WGA (red) are restricted to the mid intestine. NaPi+ enterocytes are present throughout the anterior and mid intestine, but not the posterior intestine (not shown). (F) Lateral view of a portion of the mid intestine from a 96 hpf larva that has ingested horseradish peroxidase protein (HRP). Following pinocytosis, HRP can be detected histochemically within the apical cytoplasm of specialized enterocytes of the mid intestine (segment 2) that also express NaPi (not shown). (I) Sagittal histological section through the mid intestine of the larva shown in (F), reveals HRP within the apical enterocyte cytoplasm. |
|
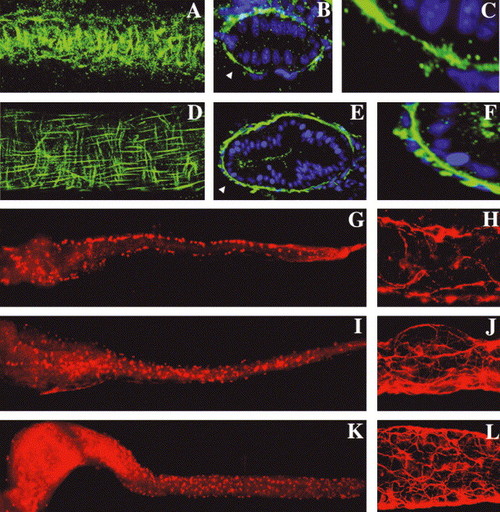
Intestinal smooth muscle development is coincident with enteric neuron differentiation: (A) Circular smooth muscle cells are first identified at 74 hpf by desmin immunohistochemistry (lateral view of a the mid intestine; whole-mount projection with anterior–posterior axis aligned horizontally). (B) Cross-sections at this stage reveal a discontinuous layer of desmin+ smooth muscle cells. (C) Enlarged inset from (B) (arrowhead) shows absence of longitudinal fibers. (D) Lateral view of the 120 hpf mid intestine, desmin immunohistochemistry; same orientation as A. Well-defined longitudinal smooth muscle cells are now evident. (E,F) Cross-sections reveal a nearly continuous inner layer of circular smooth muscle fibers and individual longitudinal smooth muscle cells peripheral to the circular muscle layer. Arrowhead points to area from E that is enlarged in (F). (G) Whole-mount view of a 74 hpf intestine processed for Hu immunohistochemistry shows enteric neuron cell bodies along the lateral borders of anterior, mid and posterior intestine. (H) Acetylated tubulin immunohistochemistry at this stage shows early axonal projections of these cells. (I–L) Hu and tubulin stainings at 96 (I,J) and 120 hpf (K,L) show proliferation and medial migration of enteric neurons and elaboration of their axonal projections. |
|
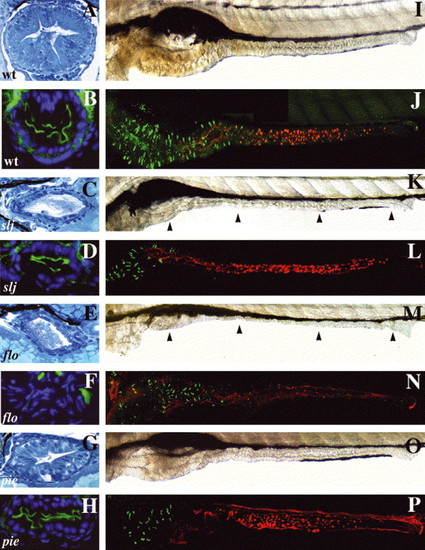
Mutations that alter epithelial maintenance also affect epithelial differentiation: (A–H) Cross-sections through the mid intestine of 5 dpf WT and intestinal mutants processed for routine histology and NaPi immunohistochemistry. (A) The WT intestine has a folded epithelium comprised of columnar epithelial cells. (B) Apical NaPi in the WT mid intestine. (C) Although the slj intestine appears undifferentiated, a similar NaPi pattern is present (D). (E) The epithelium in flo also appears undifferentiated, but lacks apical NaPi staining (F). (G) The epithelium of pie larvae is columnar with some folds and thus appears more developed than in slj or flo. (H) Apical NaPi is present in the pie epithelium. (I,K,M,O) Lateral views of the WT, slj, flo and pie intestine. (J,L,N,O) Similar views following detection of enteroendocrine cells (pancreatic polypeptide immunohistochemistry; green) and goblet cells (fluorescent conjugated wheat germ agglutinin lectin; red). (I,J) The WT intestine is folded; pancreatic polypeptide+enteroendocrine cells are restricted to the anterior intestine whereas goblet cells are located in the mid intestine. (K–P) The size of the anterior intestine and the number of enteroendocrine cells is reduced in slj (K,L), flo (M,N) and pie (O,P) mutants, although intestinal morphology is most severely altered in flo and slj compared with pie. Goblet cells appear normal in slj (L) and pie (P) but are absent in flo (N). Arrowheads point to the slj (K) and flo (M) intestine. PHENOTYPE:
|
|
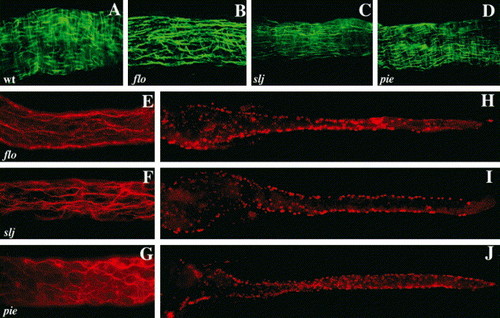
Circular smooth muscle and enteric neuron defects accompany epithelial alterations in slj and flo, but not in pie mutants. (A–D) Whole-mount view of a segment of the mid intestine of WT, flo, slj and pie larvae processed for desmin immunohistochemistry. The WT intestine (A) contains short longitudinal smooth muscle fibers and longer, underlying circular smooth muscle fibers. In the flo (B) and slj (C) intestine the longitudinal fibers predominate. By contrast, smooth muscle appears normal in pie larvae (D). (E–G) Axonal projections of enteric neurons in the mid intestine of 5 dpf flo, slj and pie larvae. Axonal projections of flo (E) and slj (F) are less complex than WT (Fig. 6L) even at 96 hpf. Axonal projections are predominantly aligned along the anterior–posterior axis in both mutants. (G) Axonal projections in pie larvae appear normal. (H–J) Whole-mount images of 5 dpf flo, slj and pie larvae processed for Hu immunohistochemistry. Compared with WT larvae (Fig. 6I,K) fewer flo and slj enteric neurons have migrated from the lateral intestinal borders, whereas the pie pattern of Hu+ cells more closely resembles WT (Fig. 6K). The number of Hu+ cells is reduced in flo and slj and fewer flo Hu+ cells have migrated to the posterior intestinal segment. PHENOTYPE:
|
Reprinted from Mechanisms of Development, 122(2), Wallace, K.N., Akhter, S., Smith, E.M., Lorent, K., and Pack, M., Intestinal growth and differentiation in zebrafish, 157-73, Copyright (2005) with permission from Elsevier. Full text @ Mech. Dev.