- Title
-
The homeobox gene mbx is involved in eye and tectum development
- Authors
- Kawahara, A., Chien, C.-B., and Dawid, I.B.
- Source
- Full text @ Dev. Biol.
|
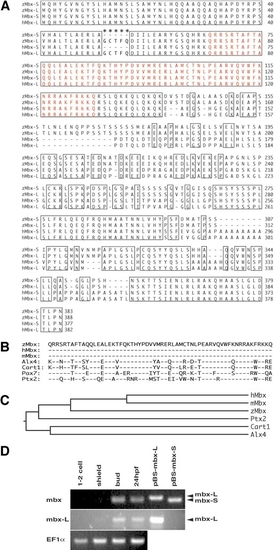
Molecular analysis of mbx. (A) Predicted protein sequence of Mbx. Identical amino acids between zebrafish (z) and human (h) Mbx are boxed, and the homeodomain is indicated by red letters. Asterisks show the five amino acids specific to the long form. (B) Sequence alignment of homeodomains of Mbx with Alx4 (Qu et al., 1997), Cart1 (Zhao et al., 1993), Pax7 (Jostes et al., 1990), and Ptx2 (Arakawa et al., 1998); hyphens indicate identity. (C) Phylogenetic tree, based on full-length amino acid sequences, including mouse Mbx (Miyamoto et al., 2002). (D) RT-PCR analysis of mbx-S and mbx-L expression in zebrafish at the indicated stages. pBS-mbx-S and pBS-mbx-L are positive control plasmids for the short and long splicing form, respectively. In the upper panel, common primers for mbx-S and mbx-L genes were used, while long form-specific primers were used in the second panel. The third panel represents EF1α loading controls. |
|
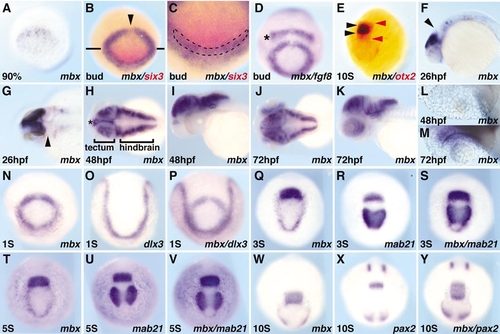
Expression pattern of mbx. Whole-mount in situ hybridization with probes shown at bottom right of each panel. Stages are indicated at bottom left, with % referring to epiboly, S to somite, and hpf to hours postfertilization. Approximate times after fertilization are 90% epiboly (9 hpf), bud (10 hpf), 1S (10.3 hpf), 3S (11 hpf), 5S (11.6 hpf), and 10S (14 hpf). Lateral view, anterior is left (F, I, K); anterior view, dorsal is up (A–E,N–Y); dorsal view, anterior is left (G, H, J, L, M). The expression of mbx is not detected by midgastrulation, but arises in the anterior neural ectoderm at late gastrula (A). At bud stage (B–D), mbx is expressed in a ring with a midline gap (B, arrowhead), and partly overlaps the ,em>six3 expression domain (C, overlap marked by dashed line). There is a gap between the fgf8 (D; asterisk) and mbx expression domains, while anterior mbx expression partly overlaps with dlx3 (N–P). During somitogenesis (N, Q, T, W), the posterior mbx domain fuses at the midline and increases in intensity, while the anterior domain elongates and then fades. Before fading, the anterior mbx domain overlaps with the eye field marked by mab21l2 (Q–S). The posterior stripe corresponds to the prospective tectum; this stripe (E; black arrowheads) is included in the otx2 expression domain (E; red arrowheads), overlaps with the posterior mab21l2 domain (Q–V), and is located just anterior of pax2.1 (W–Y). By 26 hpf, mbx is predominantly expressed in the tectum, with weak staining detected in some hindbrain and spinal neurons; mbx is excluded from the midbrain–hindbrain boundary (MHB) (F, G; arrowhead). (H–M) Expression pattern of mbx during hatching period; mbx is expressed in diencephalon (H: asterisk), tectum, otic vesicle, and rhombomeres at 48 and 72 hpf, while the retina is stained at 72 hpf, but not at 48 hpf (L, M). |
|
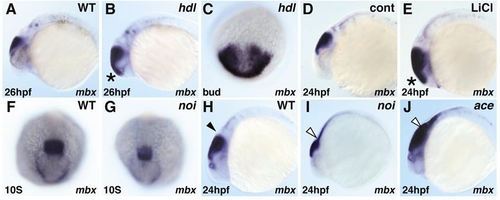
mbx expression in mutant embryos. Whole-mount in situ hybridization of mbx. Black and white arrowheads indicate the MHB and putative MHB region, respectively. In headless (hdl) mutants, mbx expression is expanded rostrally at bud and 26-hpf stages (B, C). Similarly, anterior mbx expansion at 24 hpf is observed in embryos treated with 0.3 M LiCl just after shield stage (E). In the no isthmustu29a (noi) mutant, mbx expression is slightly diminished at the 10-somite stage (G), and reduced in intensity but expanded caudally at 24 hpf (I). In the acerebellarti282a (ace) mutant, mbx expression in the tectum is retained and again expanded caudally (J). |
|
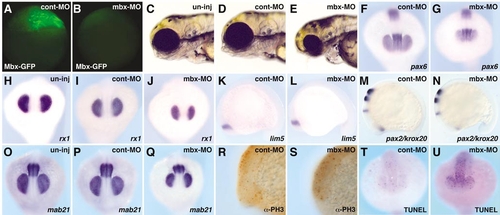
mbx knockdown phenotype with morpholino oligonucleotides. (C, H, O) Uninjected embryos; (A, D, F, I, K, M, P, R, T) cont-MO (5 ng)-injected embryos; (B, E, G, J, L, N, Q, S, U) mbx-MO (5 ng)-injected embryos. (A, B) Mbx-GFP expression at the sphere stage; lateral view. Mbx-GFP RNA (0.5 ng) was injected into the blastomere at the one-cell stage and either mbx-MO or cont-MO was injected into the yolk region. Mbx-GFP expression was suppressed in mbx-MO-injected embryos (B), but not in cont-MO-injected embryos (A). (C–E) Live embryos at 3 dpf: lateral view. Injection of mbx-MO, but not cont-MO, led to a reduction of eye size. (F–Q) Whole-mount in situ hybridization with probes shown at the bottom of each panel at the 15-somite stage. (F–J, O–Q) Anterior view. (K–N) Lateral view. Mbx-MO injection led to a smaller eye field visualized with rx1, mab21l2 and pax6 (F–J, O–Q), and a smaller tectal domain visualized by mab21l2 (O–Q). Expression of pax6 and lim5 (diencephalon: F, G, K, L), pax2.1 (optic stalk and MHB: M, N), and krox20 (hindbrain: M, N) are similar in cont-MO- and mbx-MO-injected embryos. (R, S) Anti-phosphohistone H3 antibody, visualizing mitotic cells, stains similar numbers of cells in cont-MO- and mbx-MO-injected embryos. (T, U) TUNEL assay indicates an increased number of apoptotic cells in mbx-MOinjected embryos. |
|
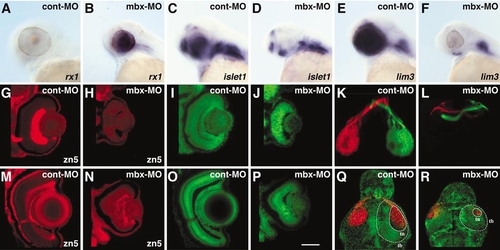
Visual system phenotype in mbx-MO-injected embryos. (A–D) Expression patterns of retinal markers at 48 hpf. The expression of rx1 in retina is down-regulated in cont-MO-injected (A), but retained in mbx-MO-injected embryos (B). Islet1 and lim3 are activated in the retina of cont-MO-injected (C, E) but not mbx-MO-injected embryos (D, F); in contrast, expression of both genes in the hindbrain is not affected (D, F). (G–J, M–P) Retinal phenotype at 48 hpf (G–J) and 5 dpf (M–P). The number of RGCs and retinal axons, as visualized by zn-5 immunostaining, is greatly reduced in mbx-MO-injected embryos (H, N) compared with controls (G, M). (G) and (H) are shown at the same brightness setting to illustrate the absence of signal in (H). Nuclear staining using SYTOX (green) shows that retinal lamination is disorganized in mbx-MO-injected embryos (I, J, O, P). (K, L, Q, R) Retinotectal projections at 5 dpf. For clarity, the labeled eyes have been digitally removed. (K, L) The right and left eyes were labeled with diI (red) and diO (green), respectively. The number of retinal axons is greatly reduced in mbx-MO-injected embryos (L), but the remaining axons project normally to the contralateral side. (Q, R) Both eyes were labeled with diD (red), and the embryo was stained with SYTOX (green), which outlines the tectal border (tb) but does not label the cell-sparse tectal neuropil (tn). The size of the tecta, and especially the neuropil, was reduced in mbx-MO-injected embryos (R) |
|
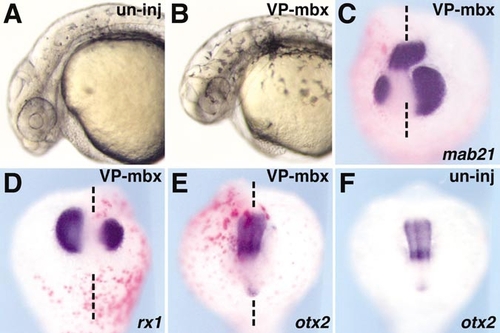
VP16-mbx expression inhibits eye and tectum formation. VP16-mbx mRNA (10 pg) together with β-galactosidase mRNA (300 pg), was injected into 1- to 2-cell-stage zebrafish embryos. (A, B) Live embryos at 25 hpf; injection of VP16-mbx caused a small eye phenotype (B). (C–F) In situ hybridization with mab21l2 (C), rx1 (D), and otx2 (E, F) at the 15-somite stage. Both mab21l2 and rx1 (purple staining) expression are smaller on the side that received the VP16-mbx mRNA injection (C, D), while otx2 expression is not affected (E). Red staining indicates the β-gal injection tracer. |
Reprinted from Developmental Biology, 248(1), Kawahara, A., Chien, C.-B., and Dawid, I.B., The homeobox gene mbx is involved in eye and tectum development, 107-117, Copyright (2002) with permission from Elsevier. Full text @ Dev. Biol.