- Title
-
Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning
- Authors
- Waskiewicz, A.J., Rikhof, H.A., Hernandez, R.E., and Moens, C.B.
- Source
- Full text @ Development
|
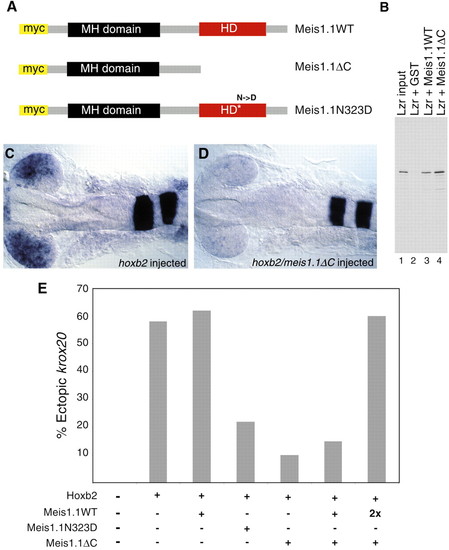
Expression of dominant-negative Meis1.1 inhibits Hoxb2 function. (A) Meis1.1WT was mutated in two alternative ways to generate forms that can bind Lzr but cannot bind DNA, Meis1.1ΔC and Meis1.1N323D. (B) To confirm that deletion of the Meis C terminus does not inhibit Pbx binding and to demonstrate that Meis1.1 can bind Lzr, the proteins were synthesized in vitro and assayed for ability to bind one another. Lane 1 contains 5% of the input Lzr, while lanes 2, 3 and 4 display proteins that bind to GST (lane 2), GST-Meis1.1 (lane 3), or GST-Meis1.1ΔC (lane 4). Binding between Lzr and Meis proteins varies from 10%-30% depending on the stringency of the wash conditions (data not shown). (C,D) hoxb2 overexpression results in ectopic expression of krox20 within the retina of approximately 60% of injected embryos. (C) 60% embryos have expression of retinal krox20 shown here at 20 somites. (D) 90% of embryos injected with hoxb2 and meis1.1ΔC contain undetectable levels of ectopic krox20. (E) Quantification of embryos expressing ectopic krox20 in the eye after injection of hoxb2 and dominant-negative meis RNAs. |
|
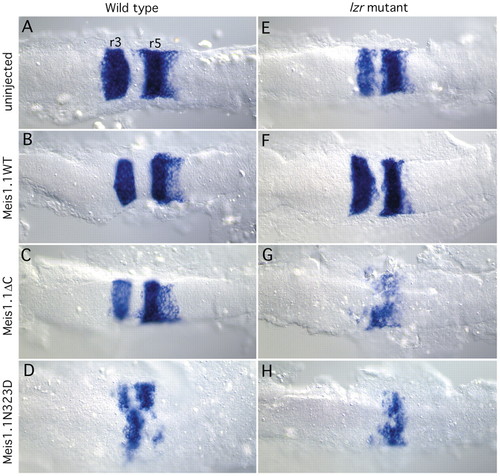
Inhibition of Meis function results in reduced krox20 expression in both wild-type and lzr mutant embryos, while overexpression of meis1.1WT partially rescues the lzr mutant phenotype. Each panel is oriented with anterior towards the left. (A-D) krox20 expression (in r3 and r5) in wild-type embryos at 8-10 somites injected with mRNAs shown on the left. Note the decrease in krox20 expression caused by Meis1.1ΔC and Meis1.1N323D in C,D, as compared with wild type in A. (E-H) krox20 expression in lzr- embryos injected as shown. Note the increase in krox20 expression caused by expressing Meis1.1WT, by comparing E,F. Genotypes of embryos were determined subsequent to photography using PCR-mediated dHPLC (Pöpperl et al., 2000). EXPRESSION / LABELING:
|
|
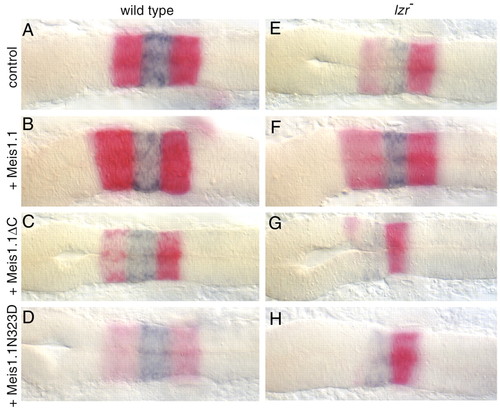
Meis activity is required for normal expression of hoxb1a, while ectopic meis1.1 increases hoxb1a expression in lzr mutants. Each panel is oriented with anterior towards the left. (A-D) krox20 expression (red in r3 and r5) and hoxb1a expression (blue in r4) in wild-type embryos at 18-20 somites injected with mRNAs shown on the left. Note the slight the decrease in hoxb1a expression caused by either Meis mutant in C,D. (E-H) krox20, and hoxb1a expression in lzr- embryos which were injected with mRNAs as shown. Note the increased expression of hoxb1a in lzr mutants injected with Meis1.1WT (compare E with F). Genotypes of embryos were confirmed by PCR-mediated dHPLC (Pöpperl et al., 2000). EXPRESSION / LABELING:
|
|
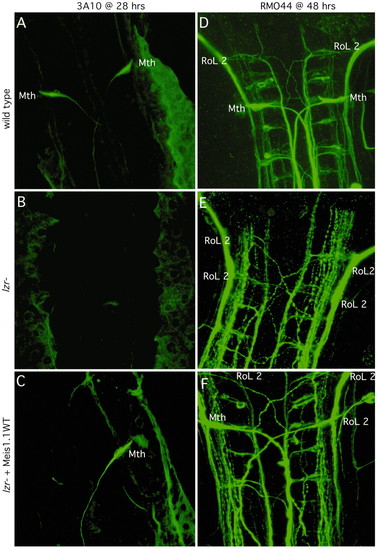
Expression of meis1.1WT in lzr mutants increases the percentage of embryos with correctly specified Mauthner (labeled Mth) neurons. Each panel is oriented with anterior towards the top. (A-C) Either wild-type or lzr- embryos were injected with Meis1.1WT mRNA as shown on left. 28 hour embryos were stained with 3A10, an antibody which recognizes the Mth neuron and its axon. (D-F) 48 hour embryos, as described on the left were stained with RMO44, an antibody that recognizes the identifiable primary reticulospinal neurons of zebrafish. Neuronal cell bodies are identified and labeled as shown (RoL 2, which is normally found in r2 of a wild-type embryo; Mth, which is in r4). |
|
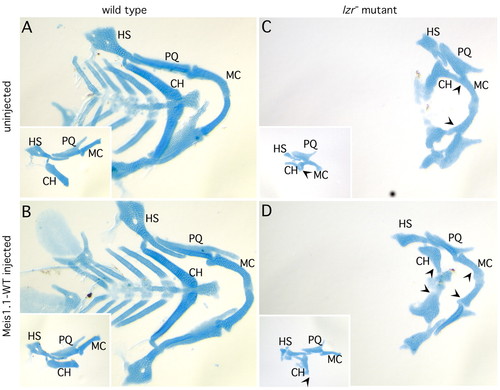
Expression of meis1.1WT in lzr mutants partially rescues jaw cartilage phenotype. (A) Alcian Green-stained wild-type embryo contains normal articulation of Meckels (M), palatoquadrate (PQ), hyosymplectic (HS) and ceratohyal (CH) cartilage elements. (B) Expression of meis1.1WT does not affect this pattern of jaw cartilages. (C) lzr mutants lack caudal cartilages, and have a prominent fusion between the second-arch derived ceratohyal (CH) and first arch-derived Meckels (M) cartilage (note arrowheads demarcating fusion). (D) lzr mutants expressing meis1.1WT have separate ceratohyal and Meckels (note the arrowheads corresponding to cartilages that are fused in panel C but not in these embryos), but do not have caudal cartilages. PHENOTYPE:
|
|
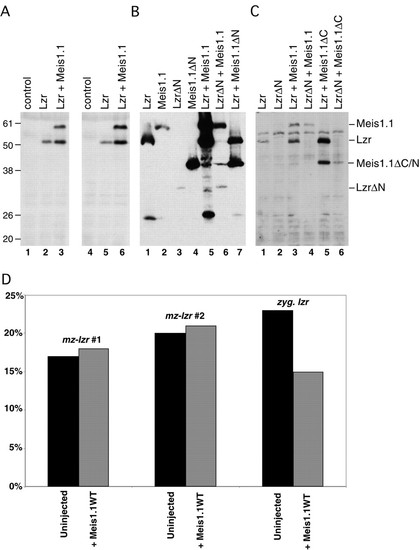
Bidirectional stabilization of Meis1.1 and Lzr. (A-C) Embryos were injected with mRNAs and lysed to isolate proteins as shown. All proteins were expressed as N-terminal fusions with the Myc epitope to ensure equal rates of translation initiation and to detect proteins by immunoblot. (A) By comparing Lzr protein levels in lane 2 and 5 (Lzr alone) with levels in lane 3 and 6 (Lzr + Meis1.1WT), co-expression of Meis1.1WT increases detectable Lzr by 3.4-fold. (B) Meis1.1 levels are increased eightfold by co-expressing Lzr, in comparison to the non-binding Meis1.1ΔN (compare lanes 1, 5, and 7). Meis levels are increased similarly by co-expressed Lzr protein, but not significantly by LzrΔN (compare lanes 2, 5, and 6). (C) Meis1.1ΔC is stabilized by Lzr and can stabilize Lzr protein (compare lanes 1, 5, and 6). Apparent molecular weights in kDa are labeled and the anticipated position of each protein is indicated. (D) Expression of Meis1.1WT does not decrease the percentage of embryos from a maternal-zygotic lzr (mz-lzr) mutant clutch with abnormal krox20. However, the same Meis1.1WT mRNA injected into zygotic lzr does reduce the percentage of abnormal krox20, indicating the ability to rescue the phenotype associated with zygotic loss-of-function. |
|
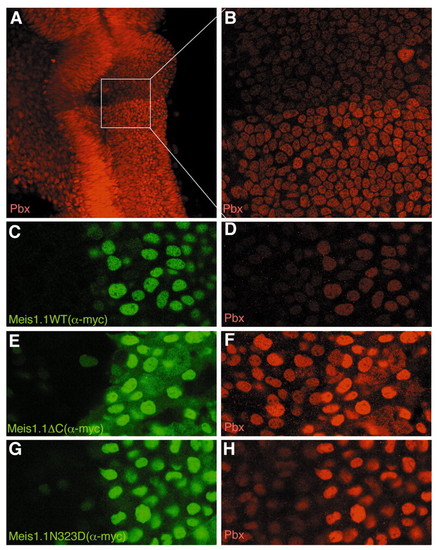
Meis stabilizes endogenous nuclear Pbx protein. (A) 24 hour embryo stained with pan-Pbx antiserum, showing a prominent boundary of nuclear staining at the r1/r2 boundary with higher Pbx levels posterior to the boundary. (B) Higher magnification image of box outlined in A, demonstrating that Pbx proteins are predominantly nuclear on both sides of the boundary. (C-H) Two to four somite stage embryos expressing Meis1.1WT (C,D), Meis1.1ΔC (E,F) and Meis1.1N323D (G,H), stained with 9E10 to visualize the Myc epitope on the Meis proteins (green staining in C,E,G) and with α-pan-Pbx antibody to detect endogenous Pbx protein (red staining in D,F,H). Note that cells expressing Meis1.1WT or mutant protein exhibit stronger Pbx immunoreactivity, whereas an unrelated Myc-tagged protein did not have this effect (data not shown). Also note that all Meis forms are predominantly nuclear. |