- Title
-
The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain
- Authors
- Begemann, G., Schilling, T.F., Rauch, G.J., Geisler, R., and Ingham, P.W.
- Source
- Full text @ Development
|
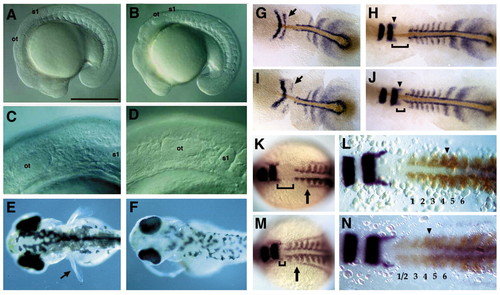
Mesodermal and fin defects in nls mutant embryos. (A,B) Lateral views of living 17-somite stage wildtype (A) and nls mutant (B) embryos, photographed with Nomarski optics, showing a kink at the head-trunk boundary in nls. (C,D) Higher magnification view of the posterior head, showing proximity of the first somite to the otic vesicle in nls (D). (E,F) Dorsal views of living 4-day-old wild-type (E) and nls mutant (F) larvae showing absence of pectoral fins (arrow) in nls. (G-N) Dorsal views of embryos labelled with in situ hybridisation and flat-mounted to show reduction in the distance between the krox20 and myoD expression domains (brackets) between nls mutants (I,J,M,N) and wild types (G,H,K,L). (G-J) Immunohistochemical colocalisation of the No tail protein (brown) with krox20, myoD and the presomitic marker her-1 in nls mutants. At the five-somite stage (G,I), expression of krox20 is slightly delayed in rhombomere (r) 5 (arrows). At the 10-somite stage (H,J), posterior head defects in nls are more pronounced and krox20 expression in r5 has recovered. Arrowheads denote migrating neural crest cells from r5 that appear normal in nls. (K,M) Co-localisation of the pronephric marker pax2 reveals that its anteriormost extension in the lateral mesoderm is located lateral to somite 3 in both wild type and nls (arrows). (L,N) Co-localisation of hoxc6 (purple) with krox20 and myoD (brown) at the ten-somite stage reveals an anterior limit of hoxc6 expression at the somite 4/5 boundary in both wild type and nls (arrowheads). Note the loss of clear separation between the first two somites in nls (N). Ot, otic vesicle; s1, somite 1. Anterior is towards the left. Scale bar: 500 μm in A,B. EXPRESSION / LABELING:
PHENOTYPE:
|
|
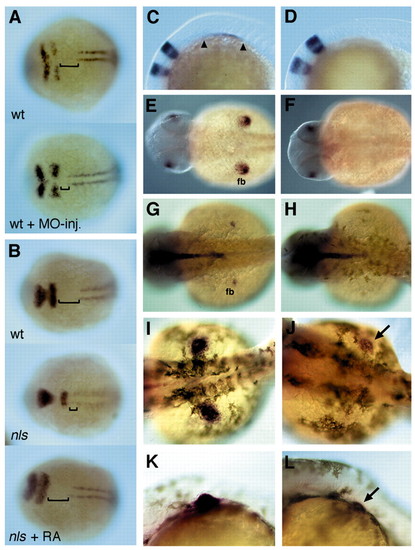
raldh2 morpholino induced phenocopies and rescue of mesodermal and pectoral fin development in nls through RA application. (A,B) Expression of krox20 and myoD, dorsal views. (A) Injection of a raldh2-morpholino into wild-type phenocopies the mesodermal defects in nls. (B) 10-6 M RA rescues mesoderm development in nls (12 hpf); brackets indicate the postotic head. (C-L) Wild-type (middle panels) and nls (right panels) embryos in dorsal (E-J) or lateral (C,D,K,L) view, anterior towards the left. (C-F) In situ hybridisation reveals an absence of tbx5.1 expression, which marks forelimb mesoderm, in nls at the 12-somite stage (C,D), as well as later during fin outgrowth at 28 hpf (E,F). (G,H) At 32 hpf, shh expression, a marker for posterior fin mesenchyme, is absent in nls embryos. (J,L) 36 hpf, 10-7 M RA rescues tbx5.1 expression in nls pectoral fin buds (arrow); rescued fin buds often develop apical folds (L, arrow), although never as progressed in growth as in wildtype siblings. EXPRESSION / LABELING:
PHENOTYPE:
|
|
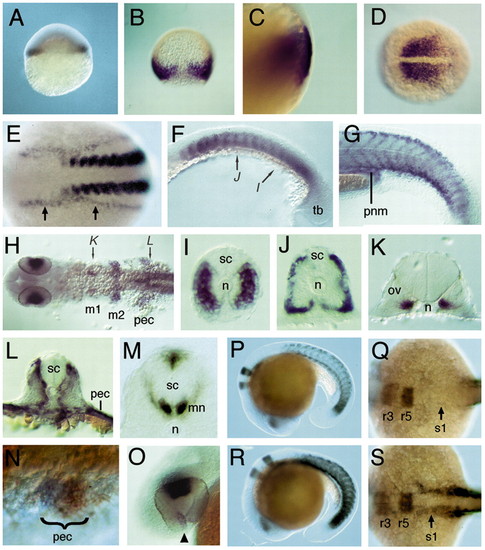
Expression of raldh2 in wild-type and neckless embryos. (A-O) In situ hybridisation to detect raldh2 mRNA in wild types. (A) Expression in marginal cells at 30% epiboly. (B) Dorsal view showing expression in the germ ring of the gastrula at 70% epiboly and absence of dorsal expression. (C) Lateral view at 85% epiboly, showing expression in deep, involuted cells of the hypoblast. (D) Dorsal view at tail bud stage showing expression in the presomitic mesoderm. (E) Dorsal view at the 12-somite stage showing expression in somites and lateral plate mesoderm (arrows). (F) Lateral view at 17 hpf showing expression in the anterior of each somite (arrows denote levels of sections in I,J). (G) Lateral view at 32 hpf, showing expression at somite boundaries, dorsal and ventral somite extremities, and pronephric mesoderm (pnm). (H) Dorsal view at 32 hpf, showing expression in the eyes and in mesenchyme flanking the otic vesicle (m1 and m2), pectoral fin buds (pec) and somites (arrows denote levels of sections in K,L). (I-M) Transverse sections at 17 hpf (I,J), 32 hpf (K,L) and 60 hpf (M), showing expression in the distal myotome but not adaxial cells (I, somite 14-level) (n, notochord), in the periphery of mature somites (J, somite 7 level), adjacent to the otic vesicle (ov) (K), and in pectoral fin and somitic mesoderm adjacent to the spinal cord (sc) (L, somite 3 level). (M) Expression in ventral motoneurones (mn) and dorsal spinal cord neurones at pectoral fin level. (N) Dorsolateral view at 30 hpf, showing expression in posterior pectoral fin buds (blue), double-stained for tbx5.1 (orange). (O) Lateral view at 33 hpf showing expression in the dorsal retina and anterior to the choroid fissure (arrowhead). (P-S) Colocalisation of krox20 and raldh2 at 19 hpf in wild types (P,Q) and in nls (R,S). (P,R) Lateral views showing upregulation of somitic expression in nls embryos, (Q,S) Dorsal views of same embryos showing upregulated raldh2 expression in lateral plate mesoderm in nls mutants (arrows); broadened krox20 expression in the hindbrain distinguishes nls from wild-type embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
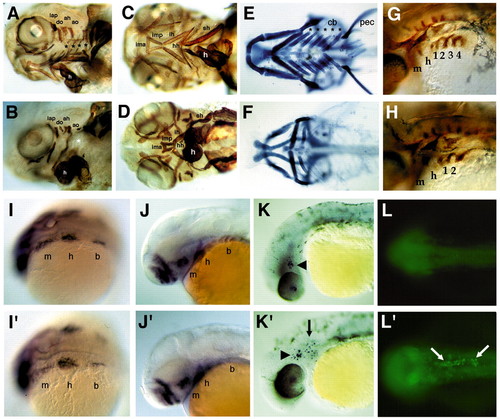
Cartilage, muscle and neural crest defects in nls. (A-D) Antimyosin immunostaining of muscles in whole-mounted wild-type (A,C) and nls (B,D) embryos at 3.5 days. As shown in lateral view (A,B), in wild types, both dorsal and ventral muscles of the mandibular and hyoid arches are present, but shortened in nls, which is clearer for the ventral muscles in ventral view (compare C with D). In branchial arches both dorsal pharyngeal wall muscles and the transverse ventral muscles (black asterisks) are absent in nls (white asterisk). (E,F) Alcian Blue staining of cartilages in whole-mounted wild type (E) and nls mutants (F) at 120 hpf, ventral views. Wild types form five ceratobranchial elements (asterisks), and in nls all but the first are deleted, as are the small hypobranchial and basibranchial cartilages in these segments at the midline. Note the absence of the pectoral fin skeleton (pec). (G,H) Immunostaining of branchial pouches with Zn8 antibody. Pouches 3 and 4 are absent in nls. Whole-mount in situ hybridisation of wild-type (I-L) and nls embryos (I′-L′′. (I,I′) Dorsolateral views of dlx2 expression in three streams of migrating cranial neural crest in both wild type and nls (m, mandibular; h, hyoid; b, branchial stream). (J,J′) Lateral views at 40 hpf showing dlx2 expression in arch primordia. Expression in the branchial arches is lost in nls. (K,K′) Lateral views at 32 hpf showing TUNEL staining of apoptotic cells in the lens and trigeminal ganglion of wild-type embryos (arrowhead), as well as in migrating neural crest cells in the branchial arches in nls mutants (K′, arrow). (L,L′) Dorsal views at 24 hpf, showing Acridine Orange staining of apoptotic cells in the anterior end of the notochord in nls (L′, arrows). ah and ao, dorsal hyoideal muscles; cb, ceratobranchial; ima, intermandibularis anterior; imp, intermandibularis posterior; ih, interhyoideus; h, heart; hh, hyohyoideus; lap and do, dorsal mandibular muscles; sh, sternohyoideus; pec, pectoral fin. PHENOTYPE:
|
|
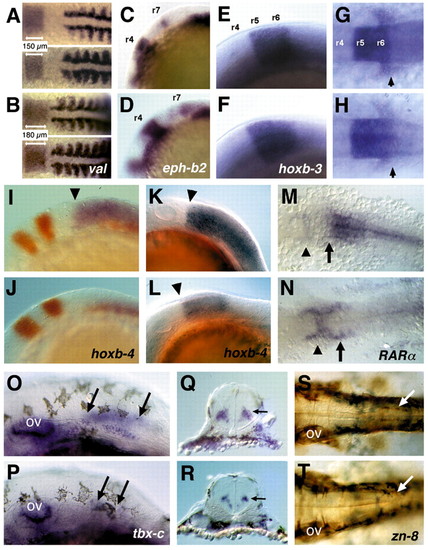
Hindbrain patterning in wild-type and nls embryos. In situ hybridisation of wild type (upper panels in A,C,E,G,I,K,M,O,Q.S) and nls (lower panels in B,D,F,H,J,L,N,P,R,T) embryos with markers expressed in the hindbrain and spinal cord. (A,B) valentino expression in r5/r6 is expanded along the AP axis (see also r3-r7 in Figs 2D,F,J); myoD expression abuts r7 in wild type and r6 and r7 in nls. (C,D) Expression of ephrin b2 appears normal in r4 and r7 in nls. (E-H) hoxb3 expression in r5/r6 and in the migrating postotic neural crest (arrows in G,H). (I-L) hoxb4 expression (blue) in the neural tube is absent in a 12 hpf nls embryo; krox20 expression (red) is expanded; myoD (red) counterstain identifies nls embryos (I,J). At 16 hpf, neural hoxb4 expression is initiated with an anterior expression boundary at r6/r7 (arrowheads), yet it is not fully expanded caudally (K.L). (M,N) RARa is expressed caudally to the r6/r7 boundary in the neural tube (arrows) in wild type; nls embryos are devoid of neural expression, while expression is unaffected or slightly elevated in more rostral regions of the neural tube (arrowheads). (O-R) tbx-c expression in interneurones of the anterior spinal cord (arrows) is reduced in nls; (Q,R) cross sections show tbxc expression in the spinal cord (arrows). (S,T) Immunostaining with the Zn8 antibody of spinal cord neurones at the level of the pectoral fins (arrows) shows loss of these neurones in nls. ov, otic vesicle. Embryonic stages: 24 hpf in A,B,E-H; 10 somite stage in C,D; 12 hpf in I,J,M,N; 16 hpf in K,L; 33 hpf in O-T. Lateral (C-F,I-L,O,P) and dorsal (A,B,G,H,M,N,S,T) views, anterior towards the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
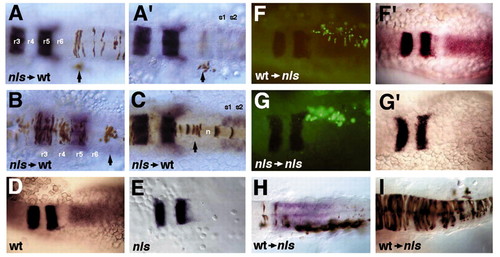
Induction of neural hoxb4 expression in nls by transplanted wild-type somitic mesoderm. (A-C) In a wild-type host nls mutant donor cells (brown) contribute to hindbrain (A), spinal cord (A,B), paraxial mesoderm (A′, arrow) and notochord (C) at the head/trunk boundary. In situ hybridisation detects expression of krox20 in the hindbrain (r3, r5) and myoD in somites (s1,s2). (D,E) krox20 and hoxb4 expression in wild type and nls mutants. (F-G) Fluorescent images of donor cells and bright field images of hosts after stained for expression of krox20 and hoxb4; wildtype cells populating paraxial mesoderm of anterior somites and individual spinal cord neurones in a 15 hpf nls embryo (F); rescue of neural expression of hoxb4 by wild-type cells (F′); nls cells transplanted to the anterior somitic mesoderm of a nls host (G) are not able to induce neural hoxb4 expression (G′). (H) Another example of rescue of neural hoxb4 expression by transplanted wild-type cells (brown staining) in somites of a nls host. Note that both sides of the neural tube are rescued in F′ and H, although wild-type cells are unilaterally distributed. (I) Example of a 15 hpf nls embryo with a massive contribution of wild-type donor cells (brown staining) to the hindbrain and spinal cord. Hoxb4 expression is not induced in either donor or host-derived neural ectoderm. Dorsal views. |