- Title
-
The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells
- Authors
- Link, B.A., Fadool, J.M., Malicki, J., and Dowling, J.E.
- Source
- Full text @ Development
|
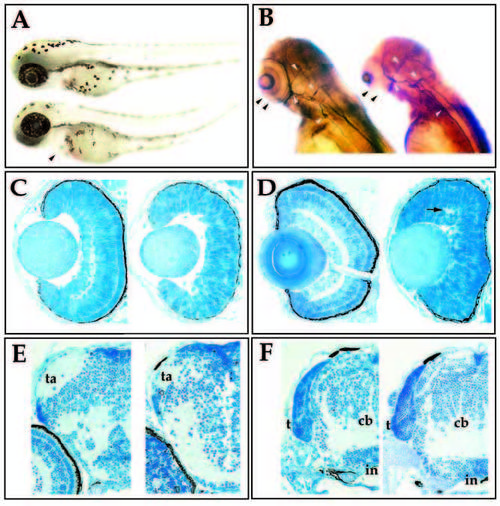
Morphological characterization of the young mutation. (A) Lateral view of wild-type (upper) and young (lower) sibling embryos at 60 hpf. Mutant embryos are characterized by swelling of the pericardium (arrowhead) and a slight curvature of the tail. (B) 3A10 immunoreactivity in wild-type (left) and young (right) siblings at 72 hpf. Mutant embryos lack retinal plexiform staining (black arrowheads), but contain 3A10- positive axonal tracts in the brain and spinal cord (white arrowheads). (C) Transverse sections of 50 hpf wild-type (left) and young (right) retina. (D) Transverse sections of 72 hpf wild-type (left) and young (right) retina. Visible lamination is absent in the young retina, although small patches of plexiform regions can form (arrow). (E) Transverse sections through the forebrain of wild-type (left) and young (right) embryos at 96 hpf. Tectal arborization fields (ta) form in both. (F) Transverse sections through the hindbrain of wild-type (left) and young (right) embryos at 96 hpf. The tectum (t), cerebellum (cb), infundibulum (in) have formed in both the mutant and in wildtype fish. |
|
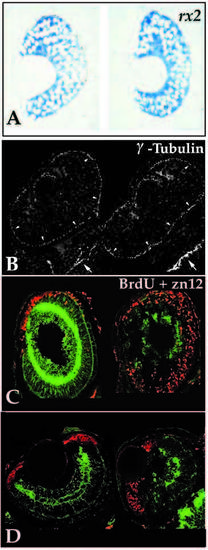
Analysis of the retinal neuroepithelium in young mutants. (A) Transverse retinal sections of 32 hpf wild-type (left) and young (right) embryos probed for rx2 transcript expression. No differences in expression patterns or levels were detected between wild-type and mutants at 32 hpf or earlier. (B) Immunoreactivity for γ-tubulin localizing the centrioles in the retina (arrowheads) and brain (arrows) of two embryos at 24 hpf. Centrioles were found to localize to apical positions in all embryos resulting from young heterozygous matings. At 24 hpf, mutant and wild-type embryos could not be consistently distinguished. (C) BrdU incorporation (red) from 62 hpf until 74 hpf when embryos were fixed for assessment of zn-12 expression. Sagittal sections, anterior up, dorsal right, of wild type (left) and young (right). In sagittal section the wave of retinal cell birthdating can be viewed in the ventral to dorsal sweep as well as from inside to outside. Note that cell proliferation has nearly ceased by 62 hpf in the wild type, while significant numbers of mutant cells destined for the outer retina, particularly in the dorsal region, continue to divide. (D). BrdU incorporation (74 hpf injection, 96 hpf fixation; red label) and zn-12 immunoreactivity (green label) in transverse sections reveal that only marginal zone cells are proliferating. Young retinoblasts (right) have exited the cell cycle similar to those in wildtype siblings (left). PHENOTYPE:
|
|
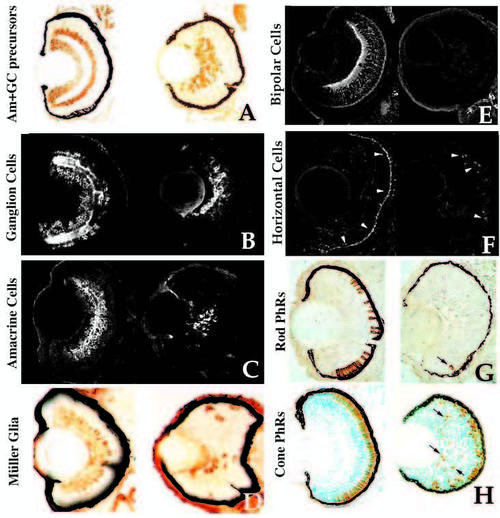
Retinal cell type marker analysis in young mutants. Central, transverse retinal sections of wild-type (left) and young mutant (right) siblings assessed for celltype- specific markers (see Text and Table 1 for details). (A) Hu antigen (ganglion and amacrine cells and their precursors), 3 dpf. (B) 7A11 antigen (ganglion cells and their axons), 3 dpf. (C) 5E11 antigen (amacrine cells and their processes), 3 dpf. (D) Carbonic anhydrase II immunoreactivity (Muller glial cells), 4 dpf. Carbonic anhydrase II is also expressed in non-ocular ectoderm. (E) Protein kinase C immunoreactivity (bipolar cells and processes), 4 dpf. (F) HCII. 7 immunoreactivity (arrowheads) (horizontal cells and their processes), 3.5 dpf. (G) ID1 antigen (rod photoreceptors), 4 dpf. Arrow indicates rod cell in the ventral patch of a mutant. (H) Zn-1 antigen (red and green cone photoreceptors], 4 dpf. Arrows indicate ectopic, mispatterned photoreceptors in the young retina. |
|
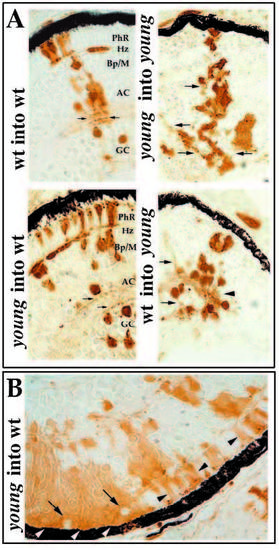
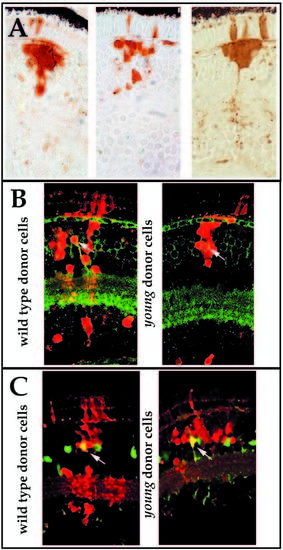
Morphological analysis of retinal cell differentiation in chimeric eyes. Chimeras were created through blastomere transplantation. Donor cells are labeled brown. (A) Donor clones in wild-type hosts (left two panels in A) that were derived from either wild-type (upper left) or mutant embryos (lower left). Photoreceptors (PhR), horizontal cells (Hz), bipolar and Muller cells (Bp/M), amacrine cells (AC), and GC (ganglion cells) each differentiate in wild-type environments and can be identified morphologically. Note the organization of sub-lamina of donor cell processes in the inner plexiform layer (arrows). Donor clones in young mutant hosts (right two panels in A) that were derived from either wild-type (lower right) or mutant embryos (upper right) are prevented from differentiating. Some neuronal processes can extend from cells of either genotype, but these axons and dendrites fail to form an organized plexiform layer (arrows). (B) Large patches of young cells in wild-type hosts show local inhibition of morphological differentiation (white arrowheads), whereas smaller clones develop normally (black arrowheads). Local inhibition is conferred on genetically wild-type (host) cells when completely surrounded by mutant cells (arrows). |
|
Some young clones in wild-type hosts show a bias for cells of the outer portion of the inner nuclear layer. (A) Examples of three such clones at 3.5 dpf. (compare to clones of 4A). (B) Protein kinase C immunoreactivity marking bipolar cells and their processes in the inner plexiform layer and in close apposition to horizontal cells and photoreceptor terminals in the outer plexiform layer (green) in wildtype (left) and young (right) derived clones (red) at 8 dpf. (C) Carbonic anhydrase II immunoreactivity marking Muller glial cells in wild-type (left) and young (right) derived clones (red) at 5 dpf. Co-localization of molecular markers with young donor cells (yellow cells indicated with arrows) demonstrates that biased mutant clones contain both bipolar and Muller glial cells. |
|
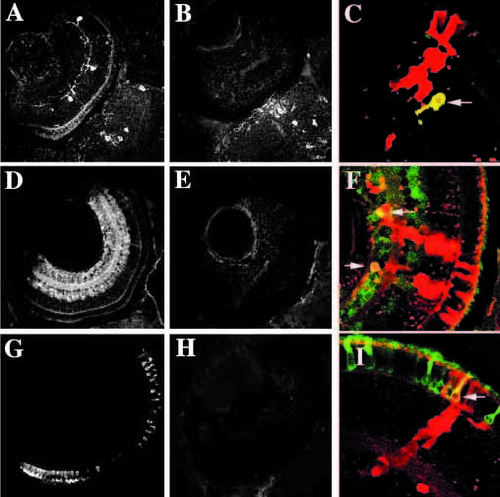
Rescue of expression of molecular markers in cells derived from young embryos when transplanted into wild-type retinas. (A-C) Tyrosine hydroxylase immunoreactiviy (interplexiform cells) in wild-type (A), young (B) and chimeric (C) eyes. In chimeric eyes, young donor cells (red) can express tyrosine hydroxylase (yellow cell indicated with arrow). (D-F) GABA immunoreactivity (amacrine cells, primarily) in wild-type (D), young (E) and chimeric (F) eyes. In chimeric eyes, young donor cells (red) can express GABA (yellow cells indicated with arrows). (G, H, I) 1D1 (rod photoreceptors) in wild-type (G), young (H), and chimeric (I) eyes. In chimeric eyes, young donor cells (red) can express 1D1 (yellow cell indicated with arrow). |

Unillustrated author statements |