- Title
-
Structure, evolution and expression of zebrafish cartilage oligomeric matrix protein (COMP, TSP5). CRISPR-Cas mutants show a dominant phenotype in myosepta
- Authors
- Forte-Gomez, H.F., Gioia, R., Tonelli, F., Kobbe, B., Koch, P., Bloch, W., Paulsson, M., Zaucke, F., Forlino, A., Wagener, R.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
Syntenic relationships of Comp gene loci in zebrafish, mouse and human. Arrows indicate the 5’ to 3’ orientation of the genes. COMP genes and syntenic genes in the direct neighborhood are in red. Note that synteny is only present downstream of the Comp genes. ZFIN gene designations are used for genes lacking a gene name. The double slash indicates regions in the human and mouse genomes (52 and 29 Mbp, respectively) lacking synteny. Note, although not in the same order and direction, the ddx49 genes in zebrafish, mouse and human are all in the vicinity of the COMP genes. |
|
Alignment of amino acid sequences of the human, mouse and zebrafish COMP. The vertical arrows mark the potential signal peptide cleavage sites (up, zebrafish; down, human and mouse). The positions of the domains are indicated by horizontal arrows. |
|
Phylogenetic tree of thrombospondins. Thrombospondin sequences comprising the EGF domains, TSP type-3 repeats and the C-terminal domains from zebrafish |
|
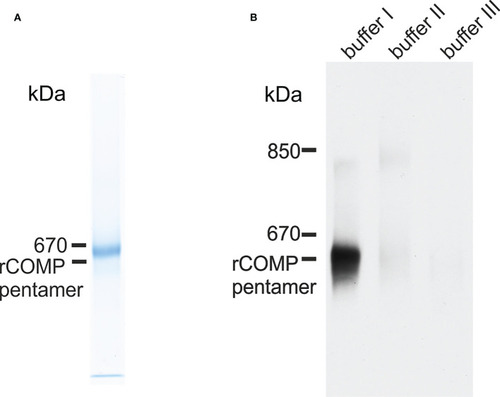
Immunoblot analysis of recombinant zebrafish Comp and Comp in extracts from zebrafish embryos. EXPRESSION / LABELING:
|
|
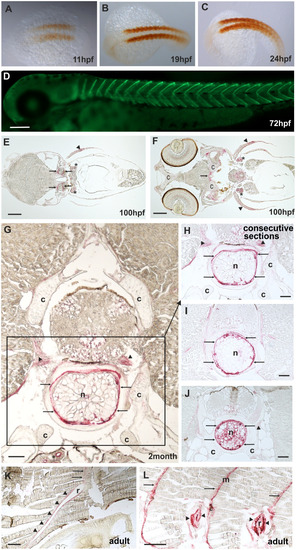
Comp distribution in zebrafish tissues at different stages of development. Immunostainings were performed using an affinity-purified rabbit antiserum directed against zebrafish Comp. EXPRESSION / LABELING:
|
|
Mutant ΔI258D259Comp is expressed in zebrafish. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Mutant ΔI258D259Comp expression in vertebral column and muscle of zebrafish. Comp immunostainings were performed on paraffin-embedded tissue sections |
|
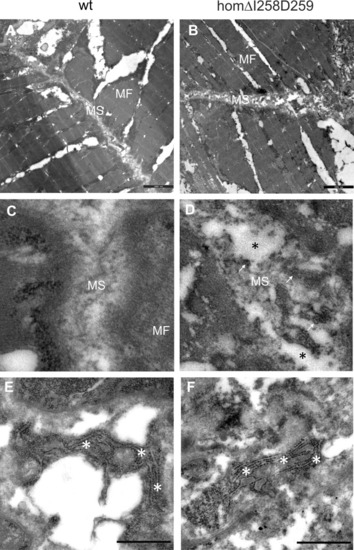
Electron microscopy of myosepta in wild type and mutant ΔI258D259Comp skeletal muscle of 72hpf zebrafish. The skeletal muscle fiber ( PHENOTYPE:
|